INTRODUCTION
It is widely demonstrated in the literature, as well as consolidated experience of every dentist, that the loss of a dental element is inexorably linked to an alteration of the alveolar bone in height and thickness, since the volume and shape of the latter are just related to the presence of the teeth (Schroeder 1986).
The dynamics and the extent of these alterations have been studied both in animal models (Cardaropoli et al 2003, Araujo & Lindhe 2005), and in humans (Amler et al 1960, Evian et al. 1982; Devlin & Sloan 2002; Trombelli et al. 2008).
These alterations vary considerably from subject to subject (Atwood 1962), but in general the clinician faces the problem to insert a implant in a site without sufficient bone support or, in any case, to find an alternative prosthetic solution which could be satisfactory also from an aesthetic point of view, despite the reduced crest.
As if this were not enough, crest reabsorption times are shorter than canonical implant insertion times in an extractive site.
Schropp and al in a 2003 study showed that the 2/3 of the reduction in thickness take place in the first 3 months following the extraction, reaching a reduction of 50% over 12 months. These data have been confirmed by Araujo and Lindhe, who published a dog study in 2005 in which comparing the dimensional alterations of the alveolar ridge following extraction of the first and second premolars showed how, despite dimensional changes could be observed during the whole year resulting in a 50% bucco-lingual reabsorption, these occur mainly in the first 8 weeks. They also found that two phases could be distinguished.
The first phase involves the reabsorption of the bone bundle with the formation of woven bone, which results in a loss of height of the buccal cortex. The second phase is represented by the reabsorption of both surfaces of the two corticals and, since the vestibular cortex is thinner than the lingual one and formed exclusively by bundle bone, this reabsorption in a horizontal direction translates into a vertical reabsorption.
Botticelli et al. confirm these data, showing a 56% and 30% vertical reabsorption in the first 4 months.
A phenomenon that all these studies highlight is the greater reabsorption of the buccal cortical, a foundamental aspect in the ridge morphology and which significantly influences the subsequent implant placement.
Currently, although the phenomenon of remodeling of the post-extraction site is well known, the causes are not yet clear. Englelr-Hamm et al in 2011 identified healing factors, and therefore in the size of the alveolus, determining factors in the remodeling of the post-extraction alveolus, since the latter is found to be exposed in the healing period at different kinds of traumas. For this reason, contrary to what could be imagined, in the molar sectors, where the alveolus is larger, a greater resorption of the bone crest is expected. These data were actually already observed by Araujo, who in 2005 had found a greater horizontal reabsorption of the buccal crest of molars (thicker) compared to that of premolars, where the buccal cortical is instead thinner
In this context, socket preservation is born as a procedure to prevent or, more properly, limit the alteration of the post-extraction bone crest as a function of an optimal implant-prosthetic rehabilitation (Jung 2009, Arauho and Lindhe 2005, Fickl et al 2008). In particular, it’s the buccal plate reabsorption to be limited, as shown in a 2008 Fickl study, where it emerges that the SP reduces on average the vestibular reabsorption of about 0.7-0.8 mm).
But what are the techniques used and what is the rationale behind them?
To answer this question, we must keep the alveolus structure in mind.
- Basal support bone that forms the body of the jaw and jaw.
- Alveolar process that develops according to the dental elements
- Bundle bone that covers the alveolus and extends coronally forming the vestibular ridge and in which the periodontal fibers are inserted (Sharpey’s fibers)
After a tooth avulsion, the alveolar bone undergoes considerable volumetric alteration. The reasons behind this process are still partly unknown, but the lack of function, reduced blood supply, localized inflammation and trauma play an important role since bone remodeling is a complex process that involves physiological, structural and functional.
We know that the healing process of the alveolar bone after an extraction is divided into two phases, in the first the bundle bone is quickly reabsorbed and replaced by woven bone with a consequent volumetric reduction in the vertical direction, in the second phase the external surface of the alveolar bone is reabsorbed causing a horizontal contraction. Socket preservation aims to limit this contraction as much as possible, as a function of implant-prosthetic rehabilitation that would otherwise be difficult or less effective (Darby et al 2009, Barone et al., 2011).
But if all the dental avulsions are followed by a volumetric reduction of the bone crest, the socket is always necessary? Actually it should be limited to specific clinical conditions, when implant insertion can not be performed in the immediate post-extraction. If the patient is not available for a post-extraction, when stability can not be obtained primary implantation, in the treatment of adolescent patients. (Hammerle and 2012)
Numerous surgical techniques have been proposed, which differ in the use of materials for grafting, membrane, for the kind of flap, but then which to use? Well, to date there is not yet a technique that unequivocally ensures success more than the others (Vignoletti and 2011). What is certain is that the different socket preservation techniques are effectively able to limit horizontal and vertical reabsorption (Vittorini Orgeas and to 2013,).
A fact that emerges from the literature is that the surgical trauma produced during the surgery could be a factor able to influence the effectiveness of the treatment, so much so that in some studies it has proved essential to limit it as much as possible in order to obtain a good result [S. Fickl et al 2008.]
In fact, in addition to the extraction phase, which for this reason should be the most atraumatic possible, it has been hypothesized that even the raising of a mucoperiosteal flap may play an important role in healing the post-extraction site since the interruption of the layer of osteogenic cells in the mature periosteum, could decrease their regenerative capacity.
Nonetheless, the results are conflicting, so that some authors report an effective reduction of the bone remodeling following flapless procedures (Fickl and 2008) while others do not find any significant difference (Araujo and Lindhe 2009, Barone and 2014)
TECHNIQUES
There are different procedures of ridge preservation. Generally they require the use of biomaterials associated with membranes (of various kinds) or the use of the membrane alone. The concept of socket preservation, in fact, is often assimilated to that of GBR, for the advantage that a barrier, allowing a selective cell repopulation, would provide. This procedure is also used in the alveoli with bone defects that require bone augmentation and has been proposed for a hypercorrection of the buccal plate, in order to compensate for the physiological contraction of the ridge with contrasting results (Fickl et al 2009).
Crestal preservation techniques can be performed with or without raising a mucoperiosteal flap. What all the techniques have in common is the need to perform the extraction in a less traumatic way, in order to preserve the post-extraction alveolus and to guarantee the stability of the clot.
In a systematic review of 2009 by Darby et al, 9 different methods of ridge preservation were evaluated. Among these, the most common was the grafting of biomaterial with membrane and flap of advancement to partially or totally cover the alveolus. The second provided for the only biomaterial grafting. The third is a membrane with a first intention closure with a flap. The other methods include different combinations of graft materials. In this study all the analyzed techniques that used membranes, provided for the raising of a flap. From the comparison of these techniques, and from the comparison with the normal healing in the control groups, it emerged with strong evidence that the ridge preservation significantly limited the reduction in height and thickness of the bone crest. It also emerged that closing by first intention was not necessary for the success of the technique, given that the results between the different methods were overlapping (in contrast to the Vignoletti study of 2012).
These results are also confirmed by a recent study on Jung’s animal, which by analyzing 4 different ridge preservation techniques with raising a flap, has observed how they, while being all effective, do not show differences in terms of bone resorption, although the same scholars admit that the cause could be identified in the surgical trauma of the periosteum, which could have limited its effects in bone augmentation techniques.
Starting from the idea that the least possible traumatism in the tissues would have further limited its reabsorption, Tarnow has devised a ridge preservation technique known as “ice cream cone technique”, literally “ice cream cone technique”. It makes use of a resorbable membrane in collagen folded to form a cone and inserted in the post-extraction alveolus, in which a FDBA graft is subsequently placed. This technique analyzed in a recent study conducted by him in 2014 would lead to a six-month average reabsorption of 1.32mm compared to the 4mm observed by Van der Weijden et al.
Among the studies in favor of ridge preservation without flaps (Fickl et al 2008), also Barone and al in 2014 showed how the flapless procedure, compared to a technique with raising a full-thickness flap, led to a reduction of the reabsorption bone especially in the horizontal sense.
On the other hand, Vignoletti et al in 2011 compared different surgical techniques had found better results in terms of horizontal resorption in cases where a mucoperiosteal flap had been sculpted. According to the authors for the success of the flap techniques, the first intention closure may have played a role, which we have instead seen not be considered an important factor in previous studies (Darby et al 2009). Other studies still do not find any difference (Araujo and Lindhe 2009).
Although the evident heterogeneity of results, all have in common a partial preservation of the bone crest. Therefore, grafting materials have been developed over time to implement the biological tissue response. Although they differ in their origin and characteristics, they all have to respect certain properties, specifically the ideal graft material should be osteoinductive, osteoconductive, biocompatible, sterile and biomechanically stable, or have the ability to maintain the space for as long as possible (Scarano et al., 2010; Brugnami et al., 1999). What is certain, given that so far no technique has yet succeeded in order to preserve the ridge completely, is that an ideal material for the socket is not available yet.
GRAFTS
Biomaterials can be classified according to the time of reabsorption in: reabsorbable, not reabsorbable and partially resorbable, or based on the origin, can be divided into biological and synthetic. In this article we will examine the biological ones.
AUTOLOGOUS GRAFTS
What is closest to the ideal grafting material is the autologous bone, today considered the gold standard for the bone regeneration, because of its osteoinductive and osteogenic abilities as well as for the total osteocompatibility. In the graft there are cells (pre-osteoblasts and pre-osteoclasts) and proteins able to induce the differentiation of the preosteoblasts. Its characteristics vary depending on the site of the sample and the type of bone used, with regard to the type of bone. It is well known as the cortical bone, made of compact bone, is characterized by periods of revascularization and subsequent longer resorption, while the medullary one, given its porous nature, is revascularized in a few weeks and reabsorbed in shorter times. Regarding the sampling site, the iliac crest bone and the marrow are the most predictable and those with the greatest osteoinductive potential. The major limitation of the autologous graft, which is also the cause of its low use, is the need of further surgery for the collection and therefore greater postoperative morbidity. The other types of grafts have the advantage of being able to be available in larger quantities without the need of second intervention on the patient, although presenting the drawback of having minor osteoinductive abilities, longer times of revascularization or to be able to transmit pathogens to the recipient. They are classified according to their place of origin in: allogeneic (FDBA and DFDBA), xenogenic (bovine or pig), alloplastic (hydroxyapatite, tricalcium phosphate, bioactive glass).
HOMOLOGICAL INSERTS (allograft)
By homologous or allogeneic grafting we mean a grafted tissue on an organism of the same species as that of origin, but genetically dissimilar. This type of grafting material is distinguished in DFDBA (demineralized freeze-dried bone allograft) and FDBA (mineralized freeze-dried bone allograft).
As for FDBA, it is biocompatible and has osteoconductive properties and is characterized by a long resorption time.
DFDBA is obtained as a result of the demineralization of bone tissue, that causes the release of BMP, the factor responsible for the differentiation of undifferentiated mesenchymal cells in osteoblasts. On this aspect the literature is divided between those who sustain that there are no big differences between the two types of grafting and, indeed, the FDBA has greater osteoconductive capacities (Piattelli A and al.1996; Yukna RA et al 2005) and those who claims that the DFDBA has osteoinductive capabilities and is therefore better than the FDBA.
Healey and Wood in 2012, for example, conducted a histological study in which, although they did not find significant differences in terms of ridge preservation, they found a higher percentage of vital bone and a lower percentage of residual graft particles in the interventions with DFDBA.
Regarding to reabsorption times, also the demineralized bone is reabsorbed very slowly, even if in shorter times than the FDBA.
However, both materials have proven effective in maintaining post-extraction bone crest, as also demonstrated by Ogihara S. and Tarnow D.P. in a 2014 longitudinal study comparing the efficacy of EMD alone and in association with FDBA and DFDBA.
In view of the undoubted effectiveness of these materials, however, it is necessary to evaluate the presence of important disadvantages such as the possibility of transmitting infections to the recipient patient, and above all the lack of histocompatibility. For these reasons allogeneic materials have been slowly abandoned, in favor of xenogenic and alloplastic grafting materials.
ETEROLOGICAL GRAFTS ( xenogenic grafts)
With xenogenic it is meant a material grafted by a species different from the human one, consequently, in order to avoid a GVHD, samples are deproteinized and consequently deprived of osteoinductive capacity. However these biomaterials show a tissue healing similar to that of homologous grafts and a high osteoconductivity. The two most used types are the bone of bovine origin and that of swine origin. Places compared, both proved effective in limiting vertical and horizontal reabsorption of the bone crest (Cardaropoli 2012)
The deproteinized bovine bone is probably the most commonly used, and over time it has proved adequate to achieve surgical success in the socket, (Artzi et al 2000, Scarano et al 2010, Cardaropoli and 2012), especially for the most long resorption times that guarantee a greater maintenance of space. In a 2009 study by Araujo and Lindhe, it emerges that it has an osteoconductive function, but not an osteoinductive function, and is able to limit crest reabsorption by acting as a scaffold for the formation of new bone. Some studies indicate an incomplete reabsorption and proliferation of connective epithelium in the grafting zones of the material (Becker et al 1998, Carmagnola et al., 2000), while others show the particles in direct contact with the bone (Artzi et al. ). Carmagnola in a 2003 study found that only 40% of the particles had direct contact with the bone, which after 6 months from extraction, still remained in the alveolus. Despite the long resorption time, Artzi in a 2001 study shows that after 9 months of alveolus residue, no connective tissue was found in contact with the granules, this demonstrate that the DBBM is a biocompatible material suitable for socket preservation procedures in which longer resorption times are preferred. In fact, the slow rate of reabsorption of some biomaterials is clinically an important advantage since they contribute to stabilize the site, in contrast to autologous bone, with which it is found a high rate of resorption compared to the original volume (Sbordone and 2011) . By contrast, Carmagnola et al in 2003 in a very similar study in which DBBM was used as grafting material, had observed that the biomaterial particles persisted over 6 months after extraction and that only 40% were in direct contact with the bone.
From the literature it emerges that there are no univocal results regarding the behavior of biomaterials, on which very different results have often been obtained. Furthermore, another difficulty is represented by the identification of data in the literature that are effectively valid and not affected by bias, given the limited amount of well-designed studies (Vignoletti 2011),
SUMMING UP
Next, a series of socket preservation cases performed with three different techniques are shown. Whatever it is the technique, it is important that the extraction is as atraumatic as possible. We must avoid leverage the alveolar bone. For this reason, in cases where we have to remove an apex, we can use appropriate tools that allow us to extract the root without damaging the surrounding structures. There is also the possibility of using endodontic files to help us in these extractions.

Bio-Oss Collagen + Mucograft
It is a heteronomous bone substitute consisting of 90% of deproteinized bovine bone particles and 10% of porcine collagens with the function of making the material more easily manipulated. Some studies (Orsini G and in 2005, Jung RE and in 2013) have shown their osteoconductive properties and a good integration in the neo-bone. Moreover, thanks to its slow reabsorption, the material is volumetrically stable (Araujo MG and in 2010, Mordenfeld A and 2012).
Mucograft. It is a 3D collagen matrix designed as alternative to the withdrawl of connective tissue. It’s composed of a compact external layer and an internal spongy layer that provides stability to the coagulation allowing tissue repair. The autologous tissue is in fact extremely effective but provides a troublesome procedure for the patient, while, as shown by some studies, mucograft significantly reduces postoperative morbidity (Sanz M and at 2009, Lorenzo R and at 2012) while reducing the duration of intervention (Sanz M and 2009, Cardaropoli D and 2012). Among the advantages brought about by this matrix there seems to be a reduction in healing times with respect to the connective tissue (Thoma D and 2012), as demonstrated also in a study by Mesceva and in 2018 in which the tissue is histologically evaluated at six months from The intervention showed a significant difference between the patients treated with free graft who had a fragmented collagenous tissue and among the patients treated with mucograft, who instead presented a mature tissue.
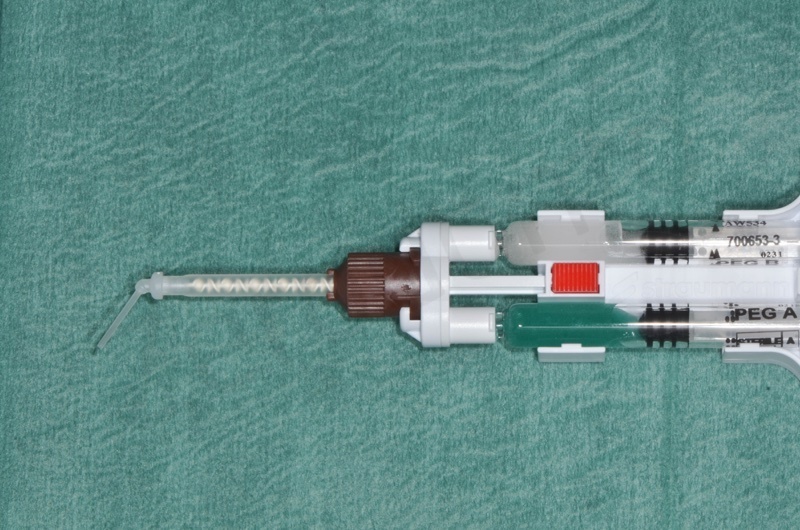
MembraGel. It is a gel membrane based on polyethylene glycol to be applied with a special dispenser for direct mixing of two synthetic components, which solidifies in a minute stabilizing the biomaterial granules in their most superficial layer.
Case Report 1
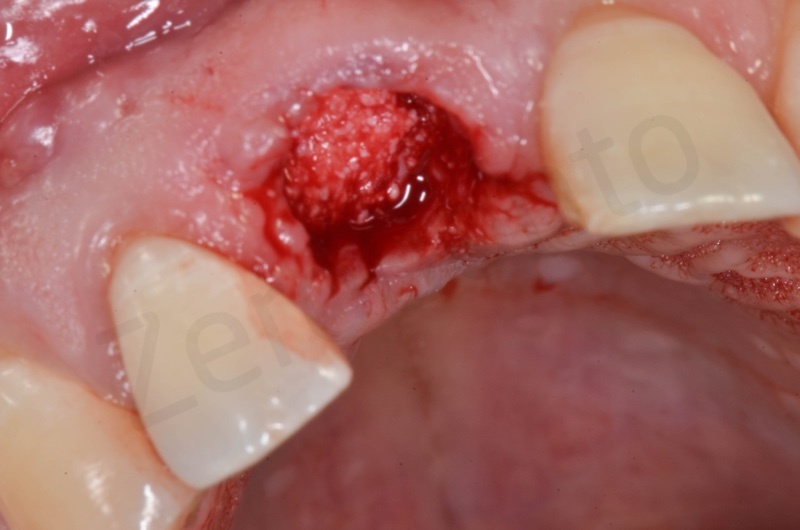
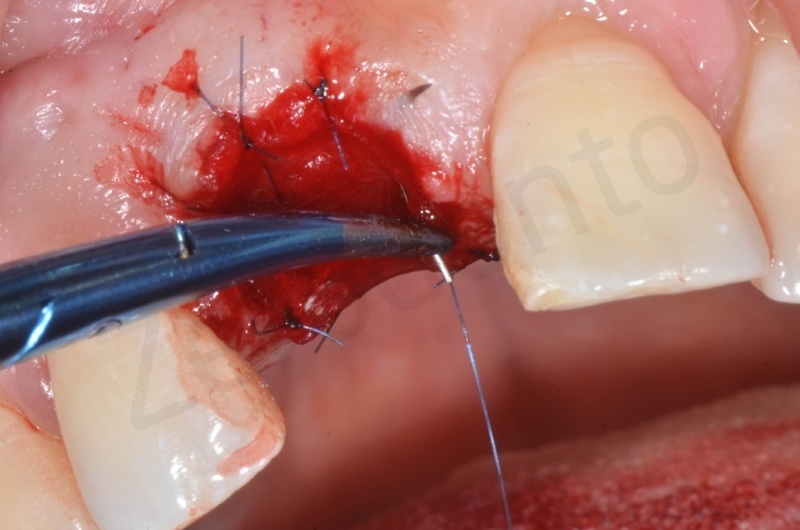
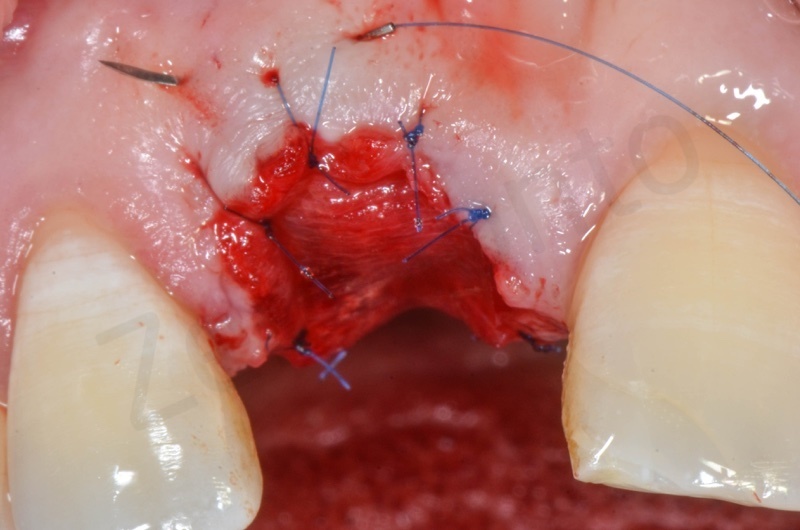
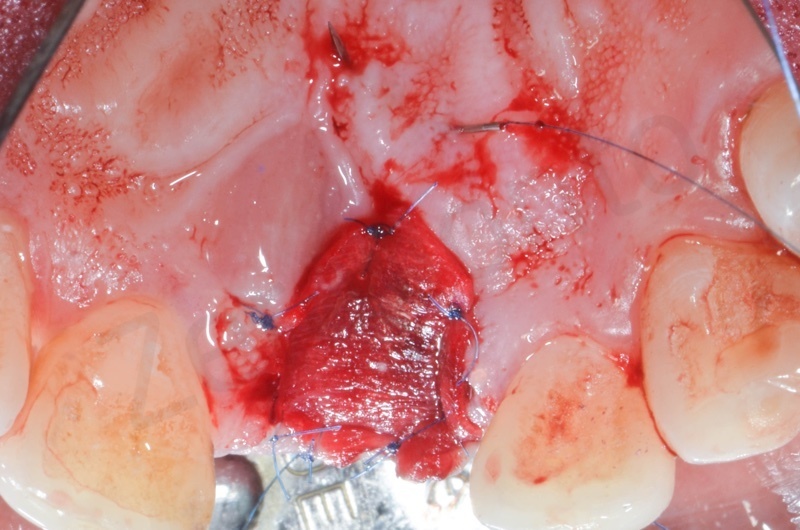
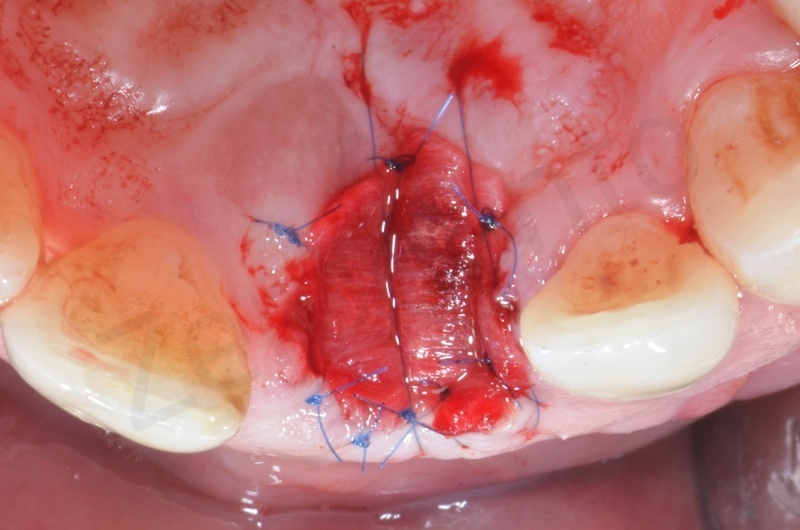
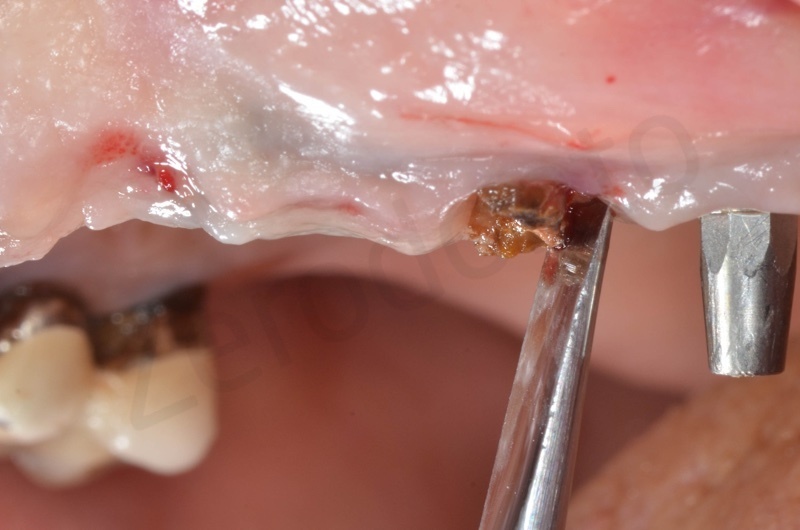
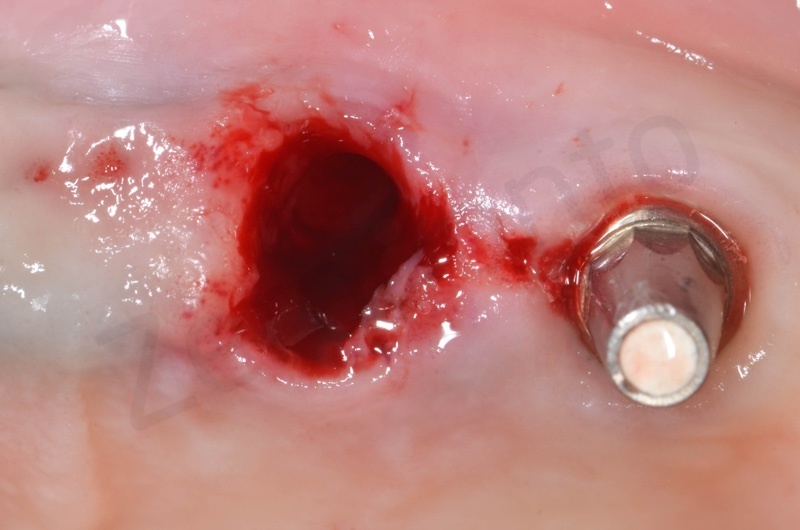
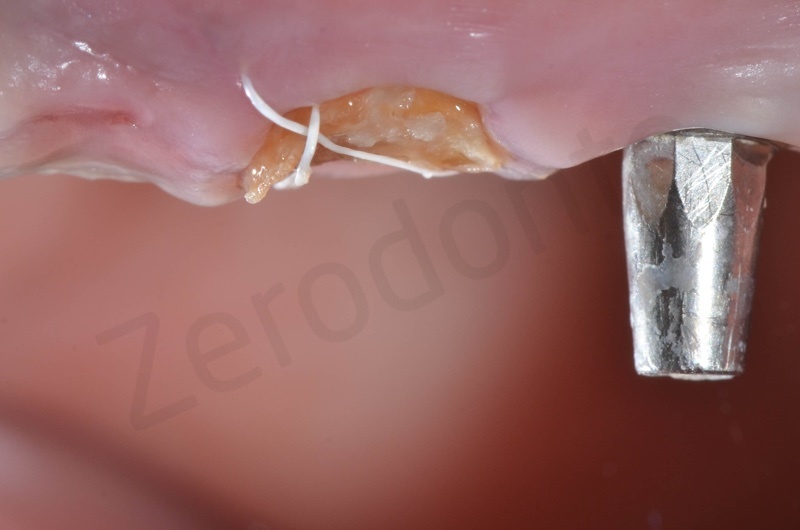
Atraumatic avulsion with the help of an elevator and a forceps.
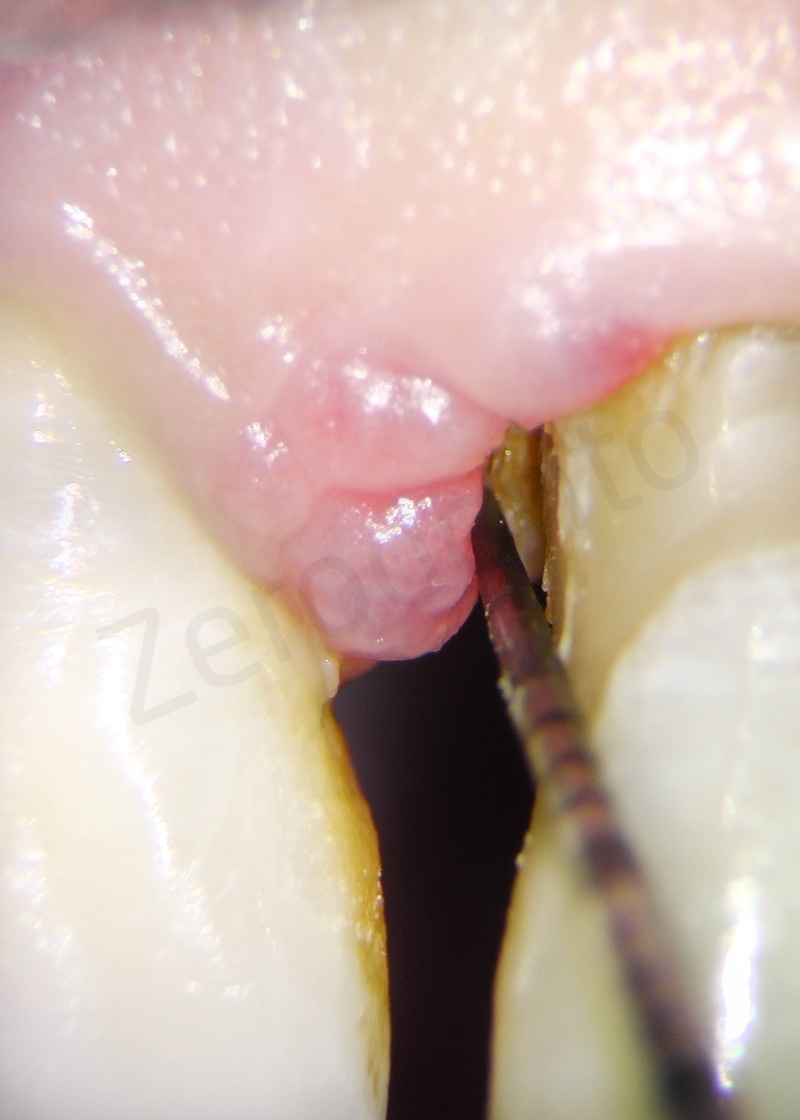
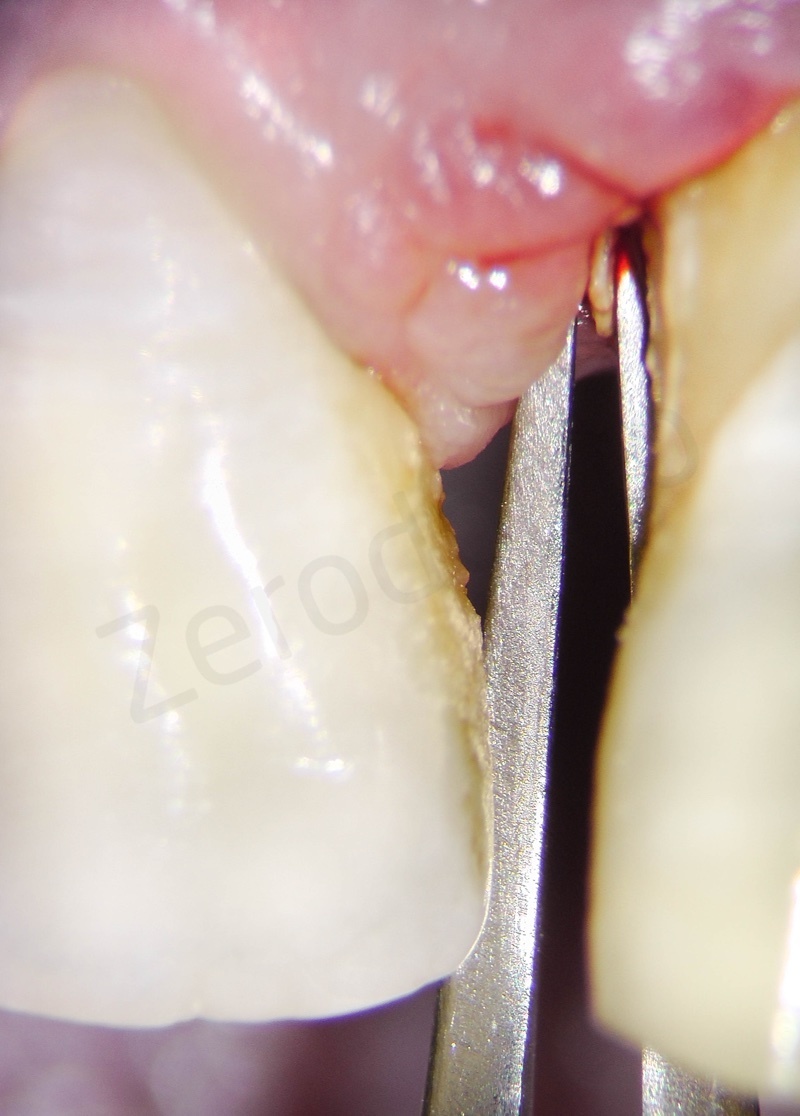
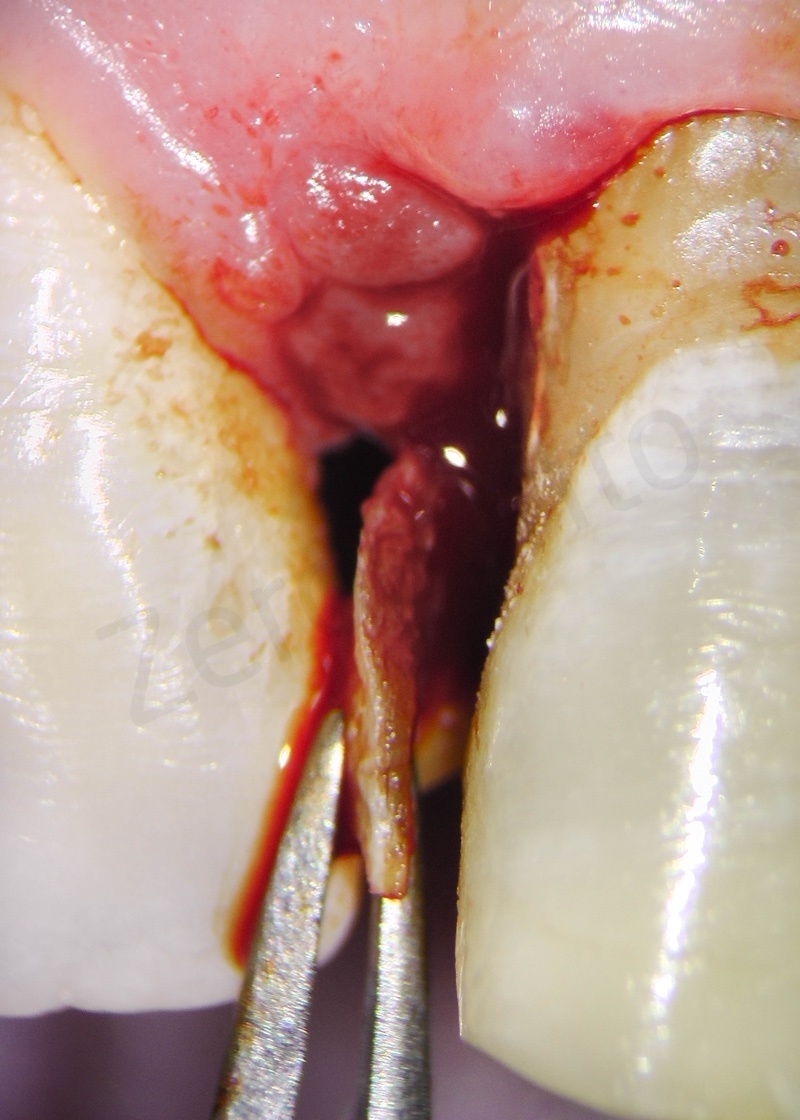
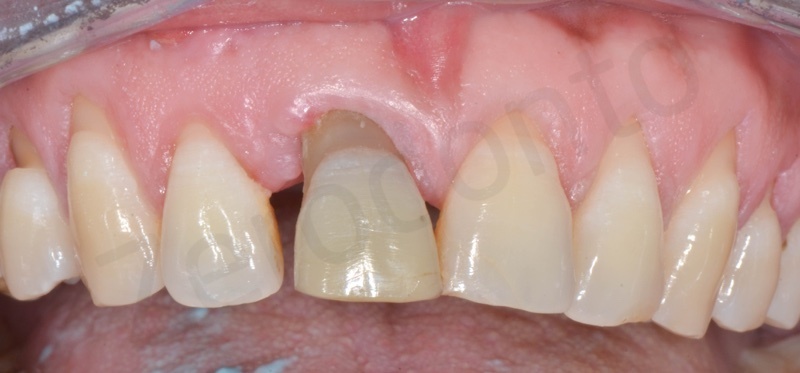
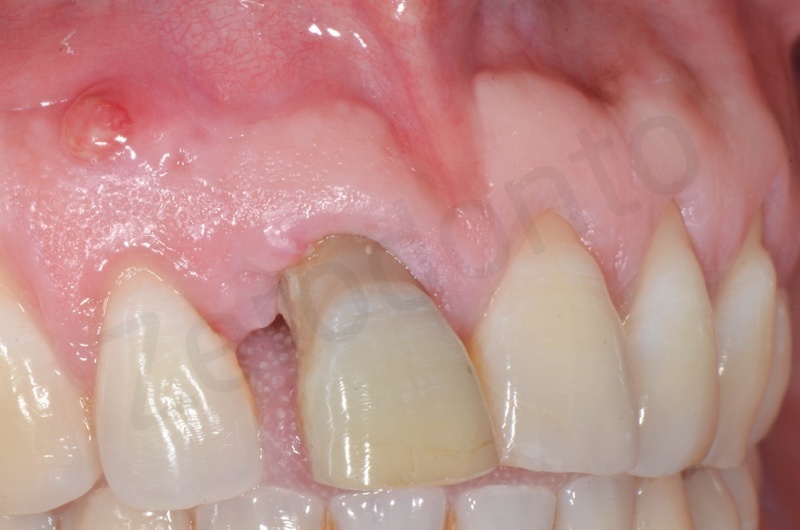
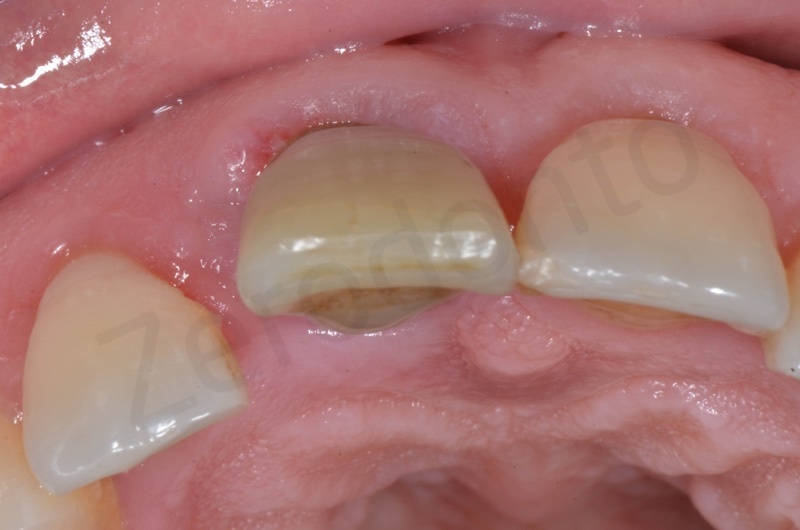
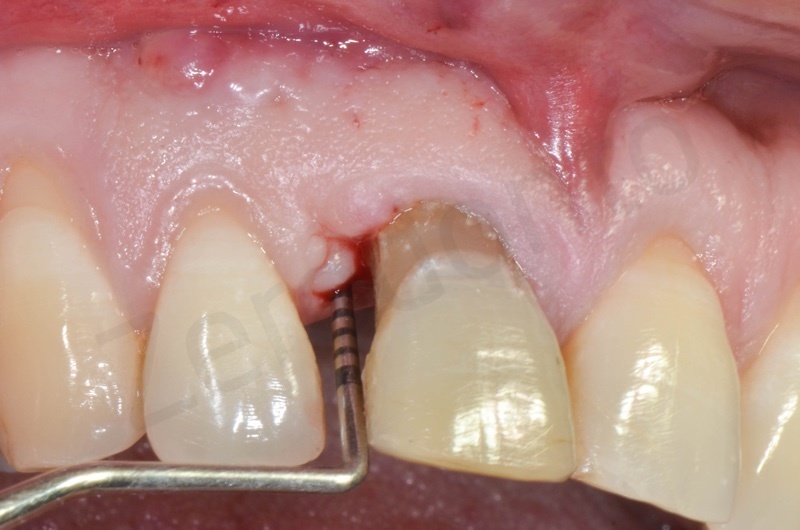
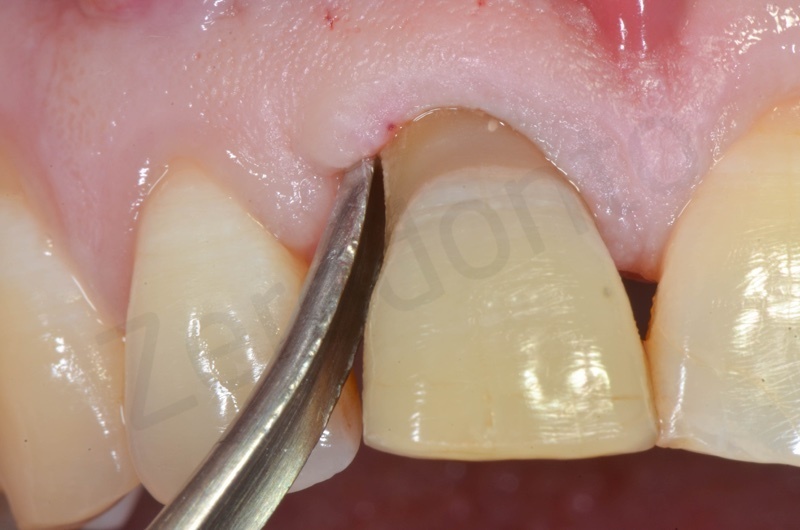
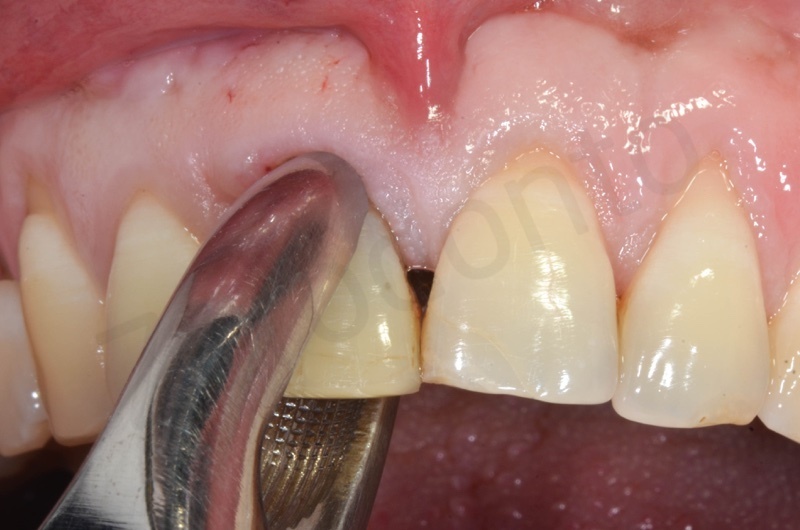
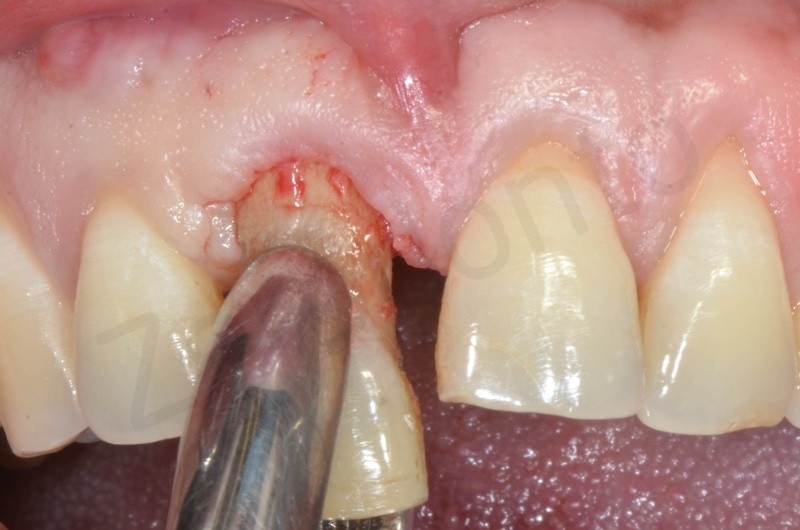
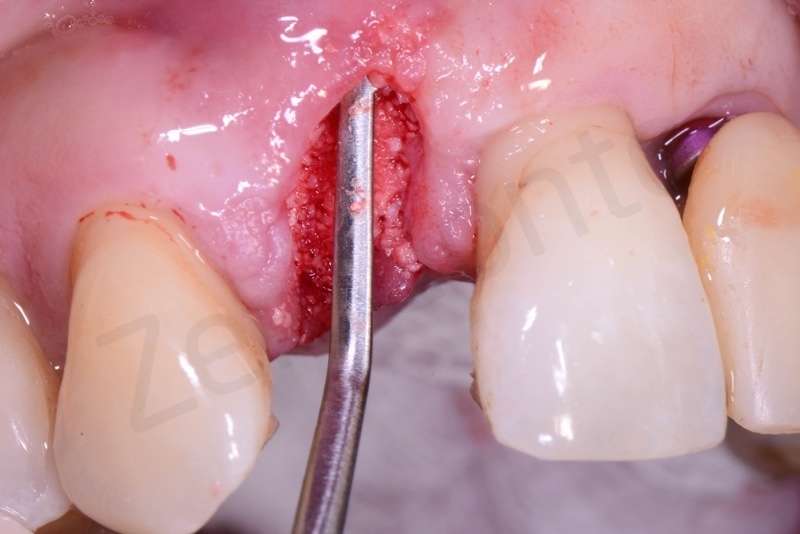
Disepithelization of the socket performed with a scalpel 15c.
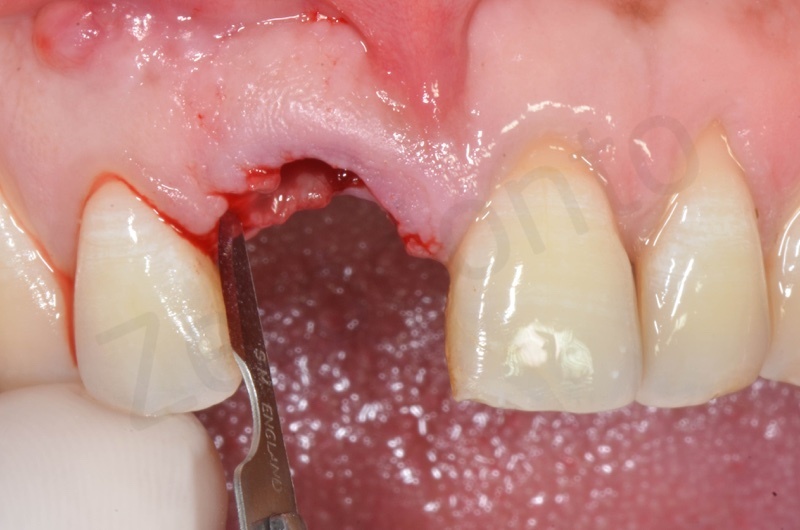
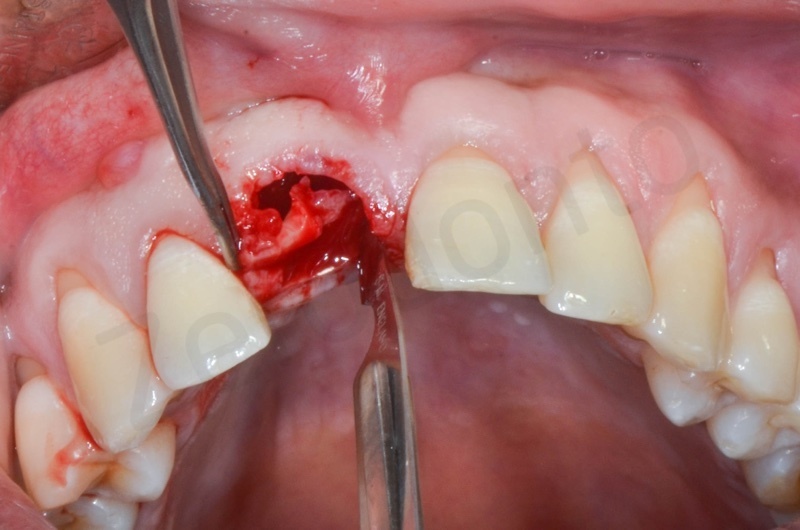
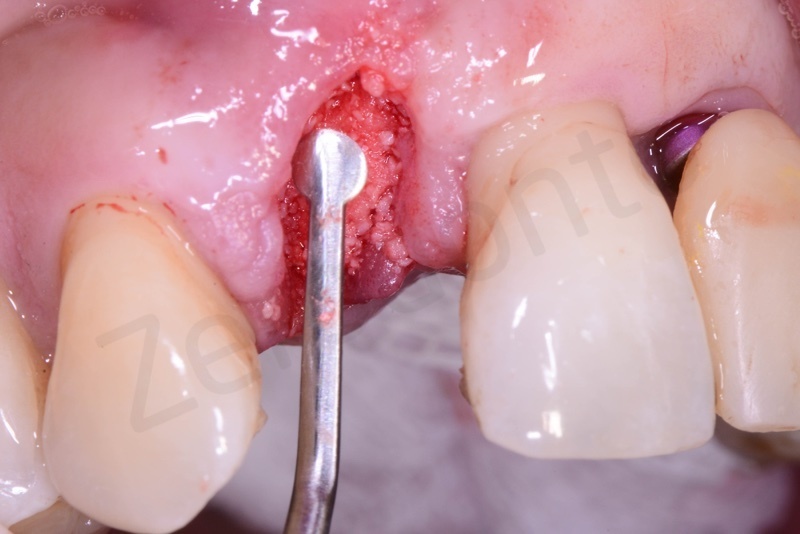
Curettage of granulation tissue.
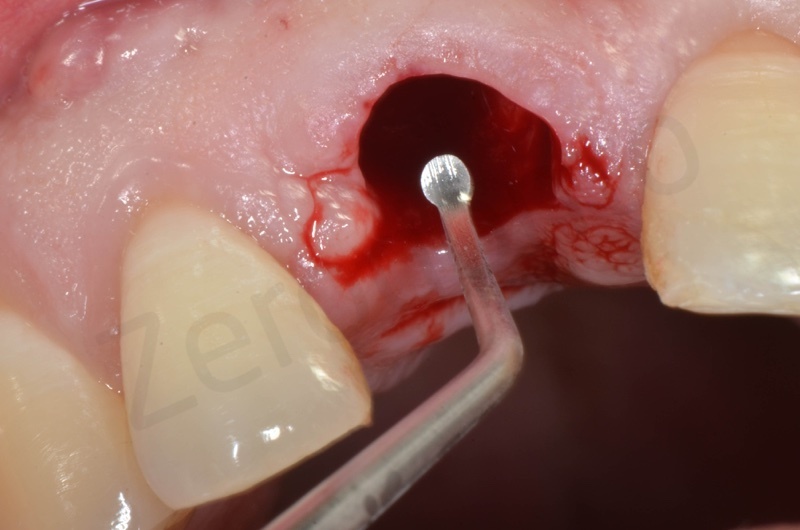
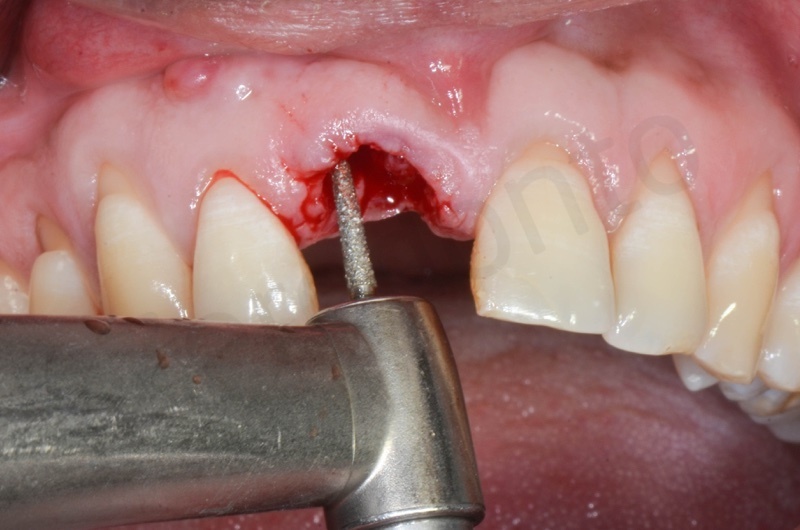
Disepithelization by means of a low speed diamond bur.
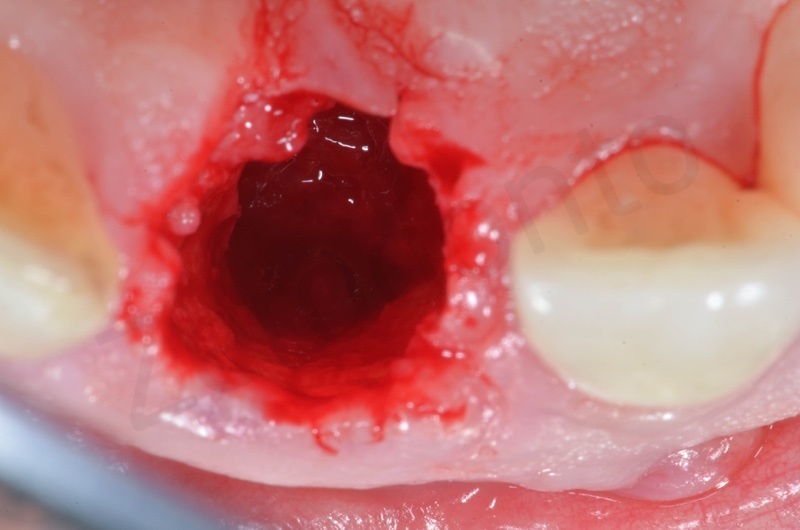
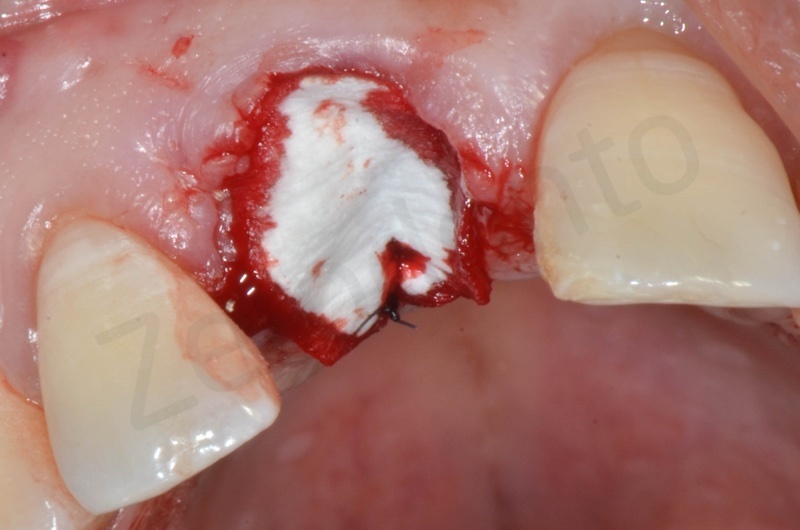
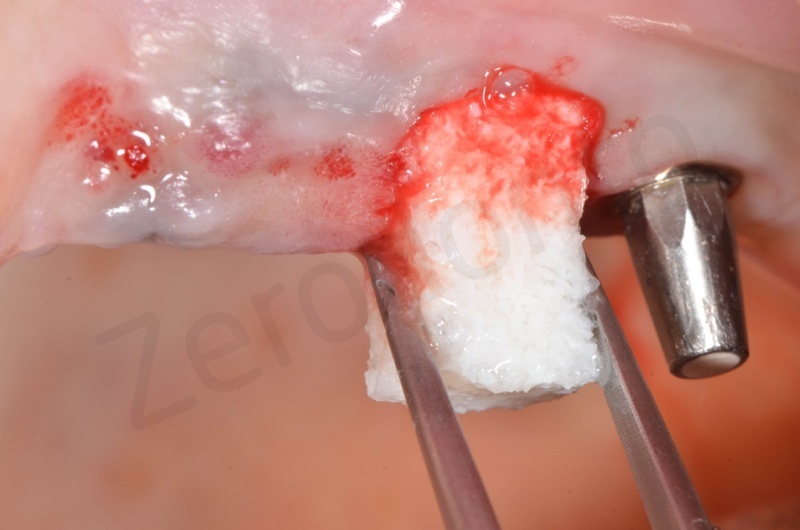
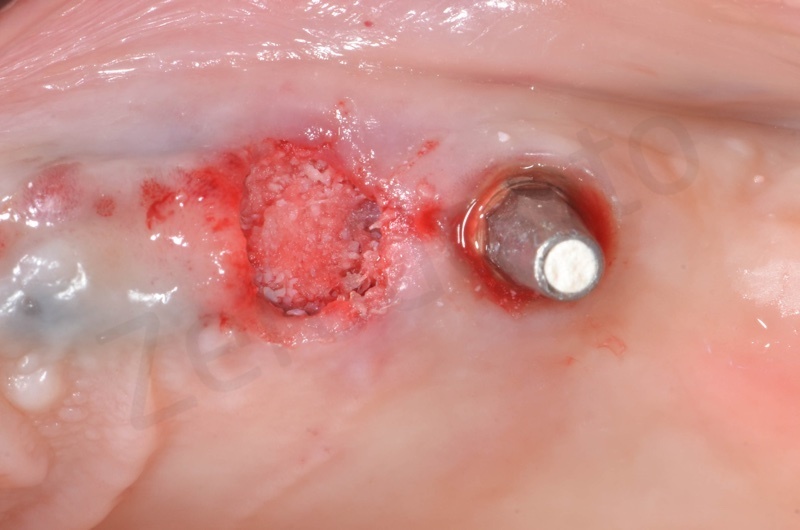
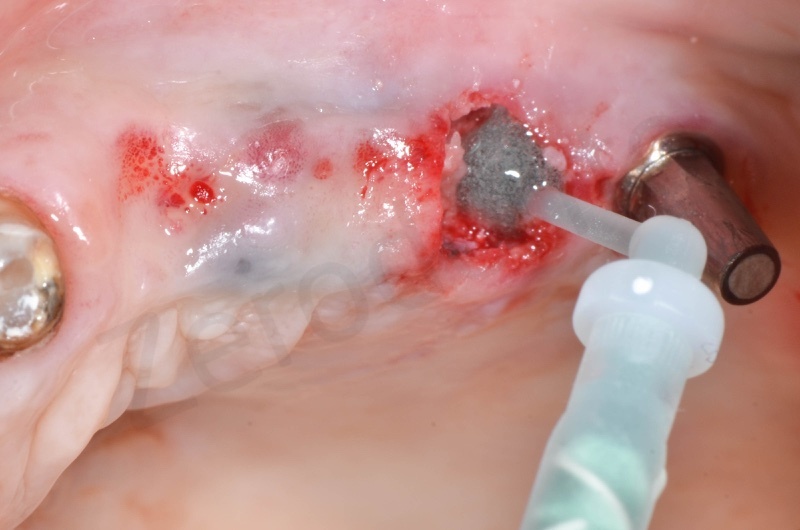
Bio-Oss collagen insertion.



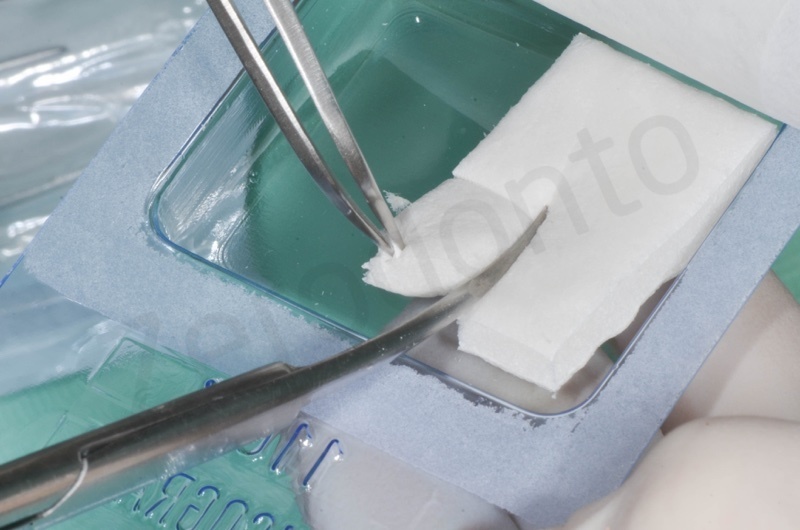
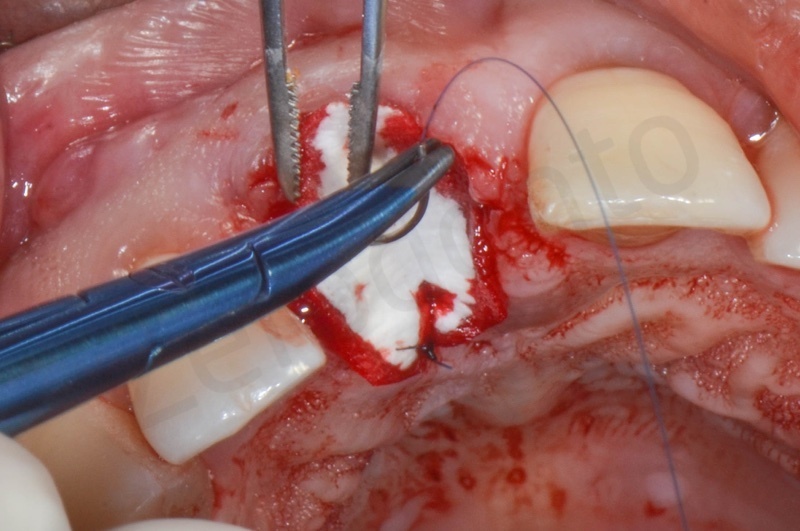
Mucograft application, suturing.
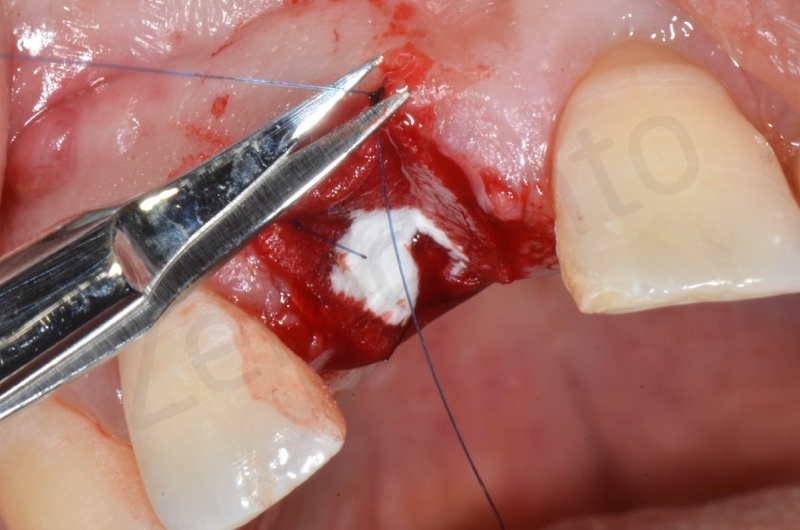
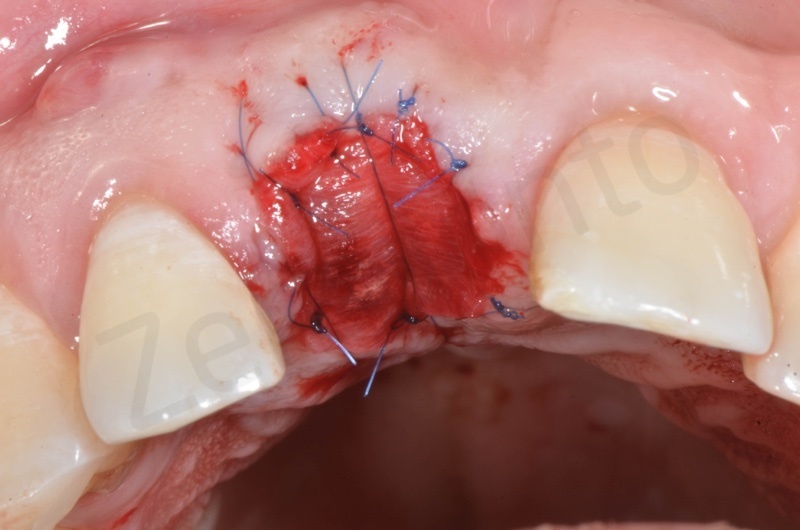
A maryland guided tissue healing provides also a good post surgery esthetics.
1 week post-op.
Case report 2
Female, 68 years old, no smoker.
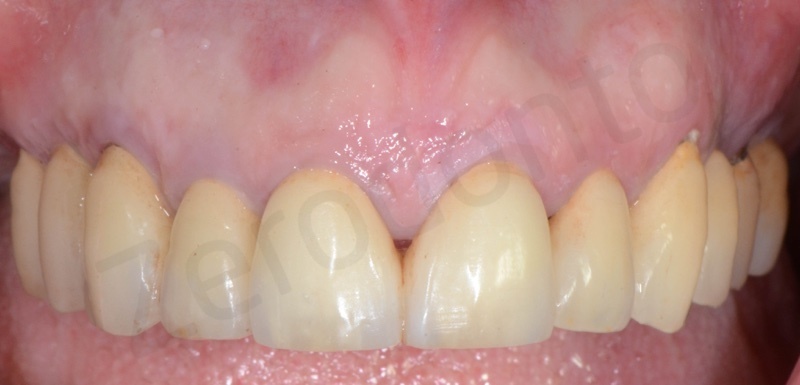
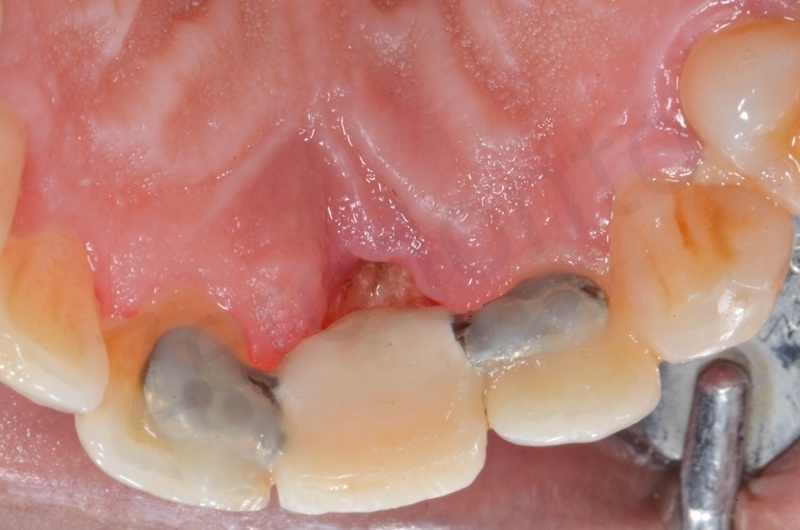
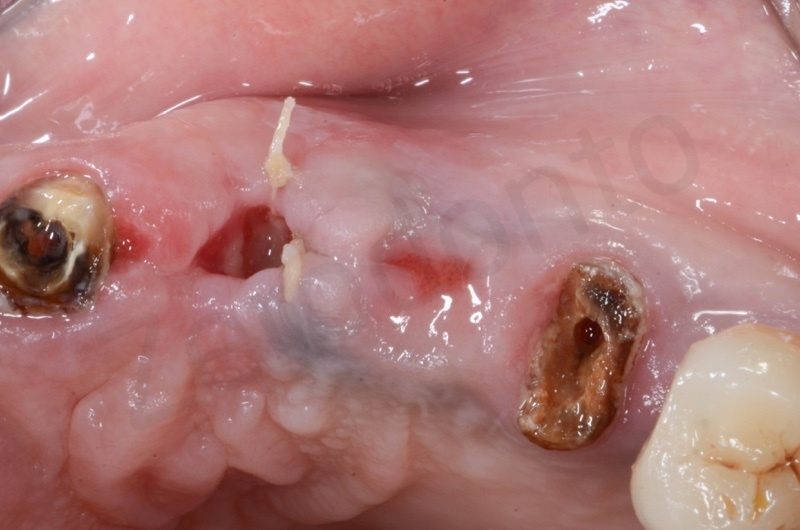
Upper arch with root of teeth # 22, 23, 25, where 22 and 25 will be the foundation of a 22-25 bridge.
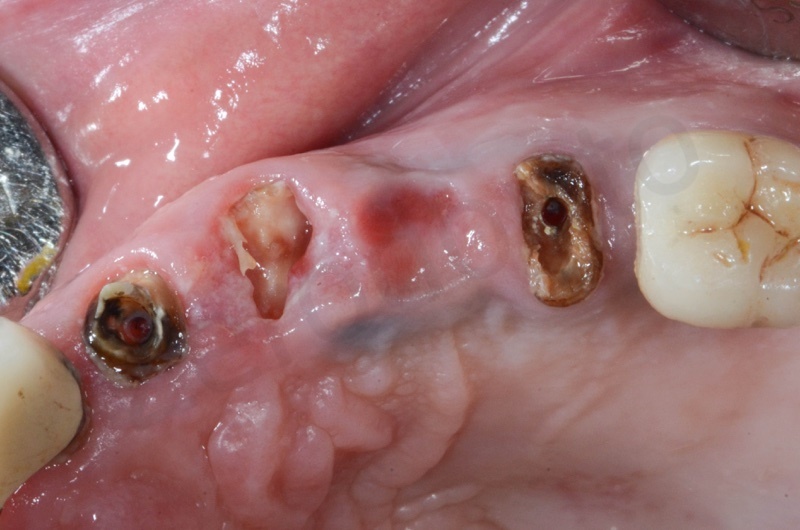
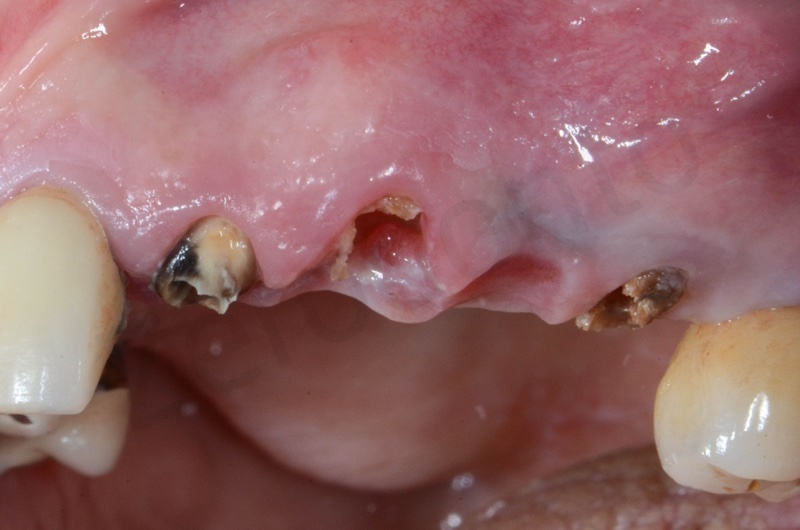
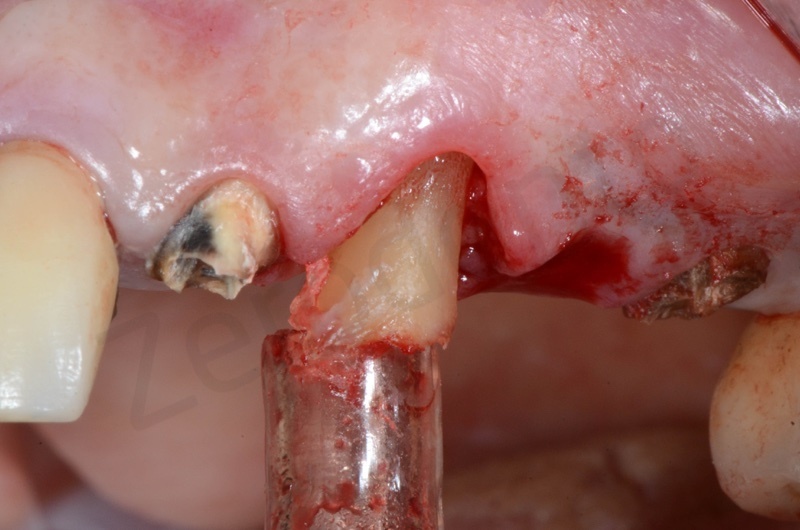
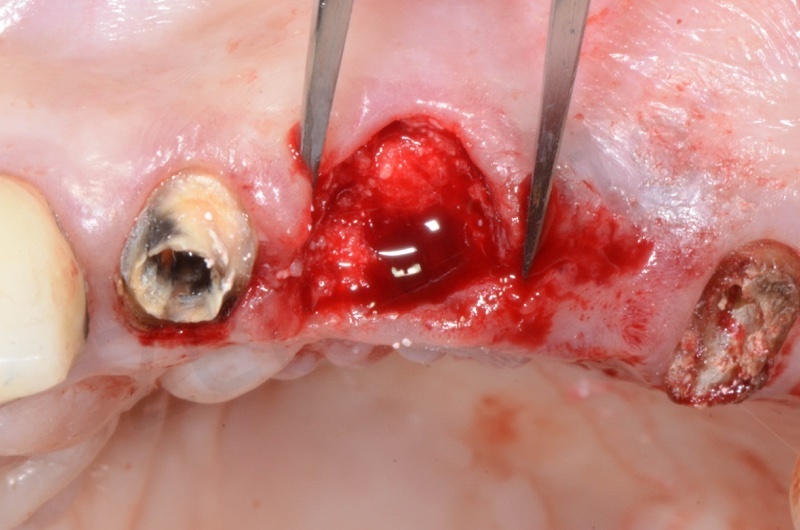
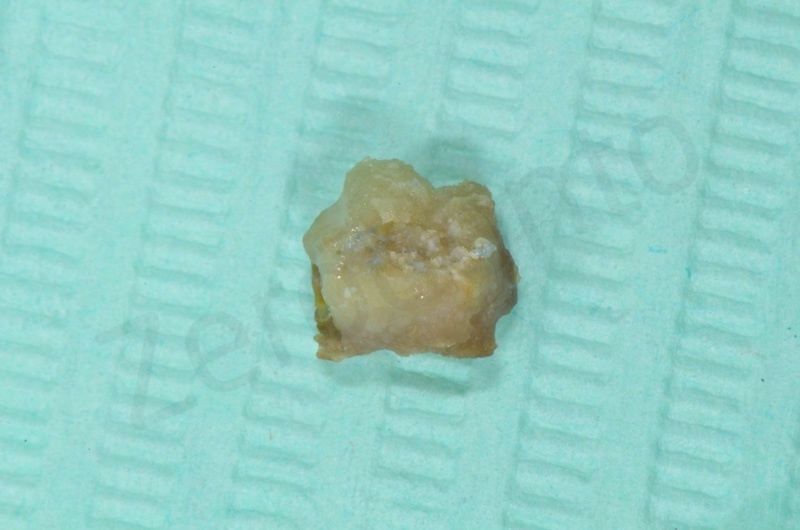
The root of the #23 is extracted, granulation tissue and sulcular epithelium are removed. #22 and #25 will be successively removed.
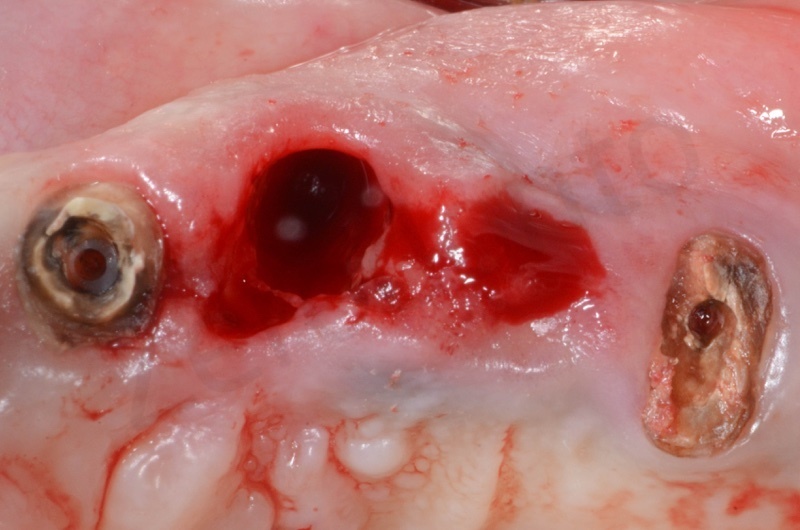
Bio-Oss collagen is moisturized with saline solution.
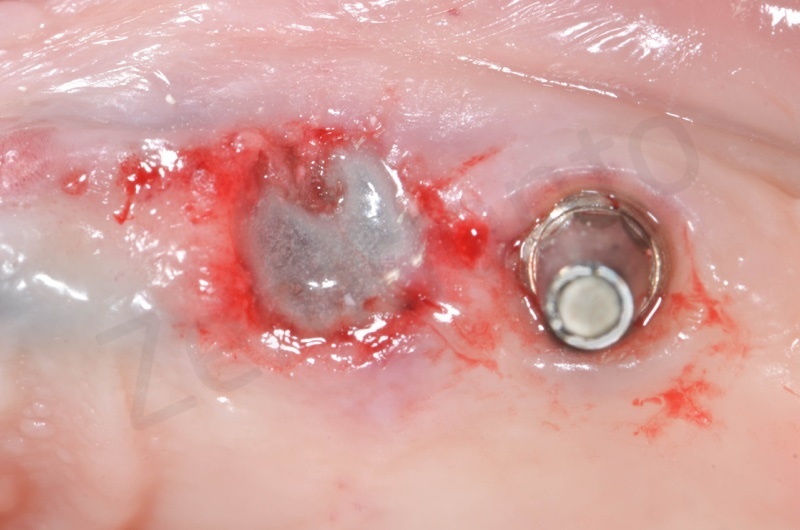
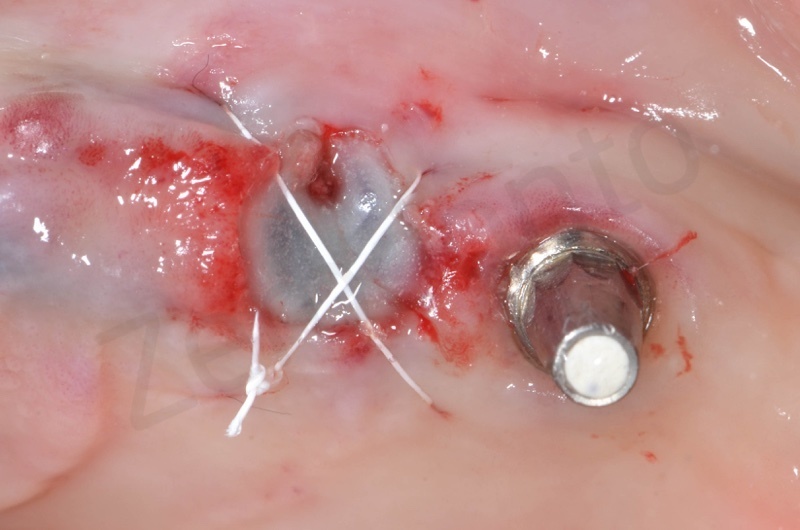
Bio-Oss collagen inserted in the alveolar socket
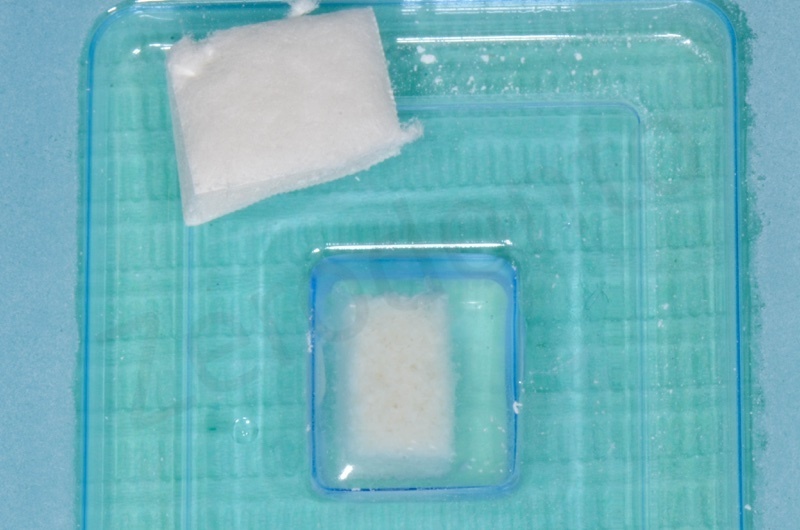
Socket’s dimension will determine Mucograft size.

Dimensions on Mucograft’s package.
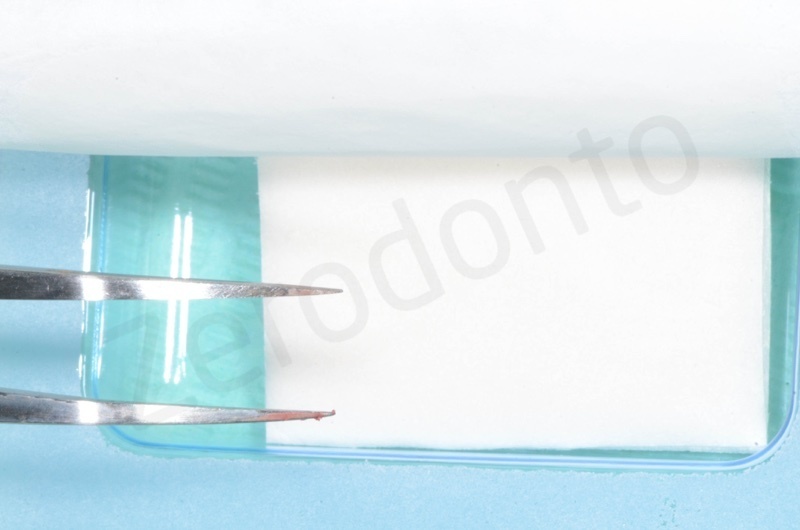

Details at microscope


Matrix is positioned and anchored with suture.
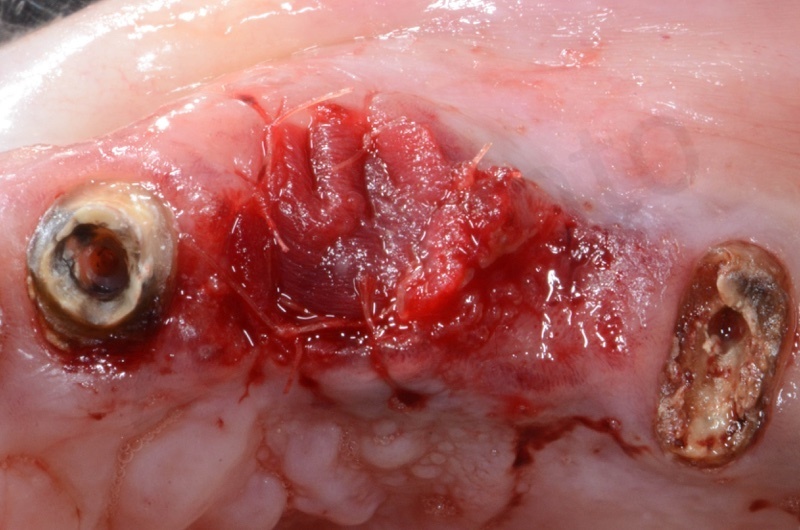
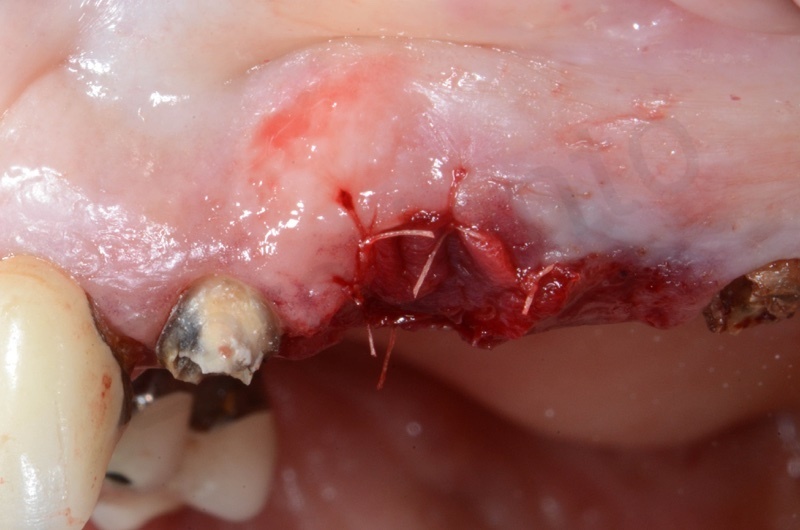
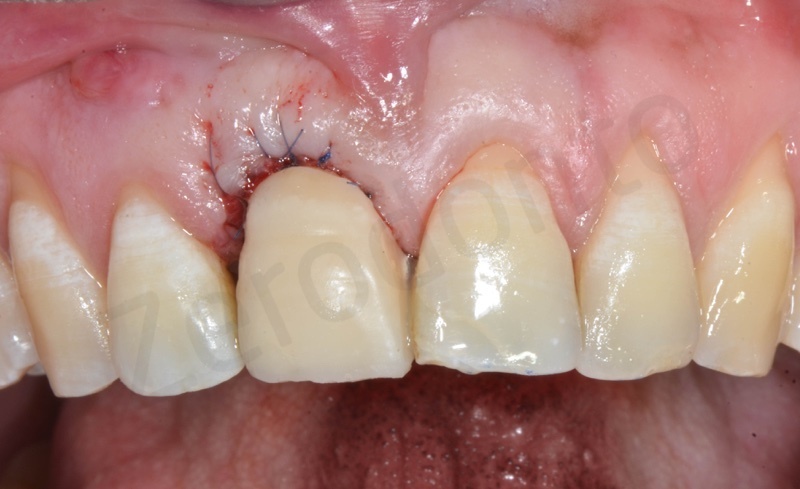
Healing after 1 week.
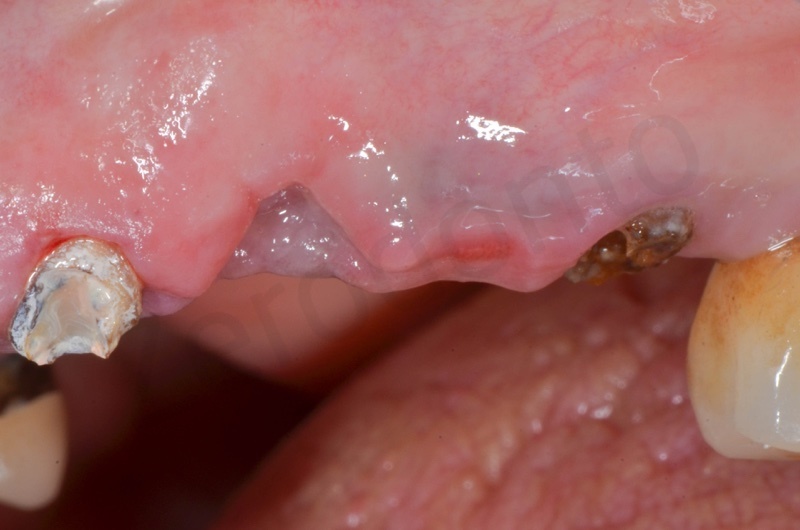
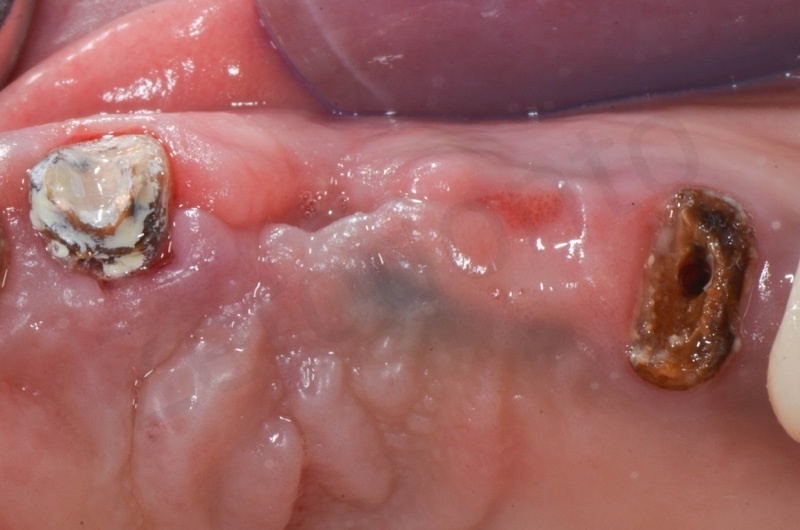
After 1 month.
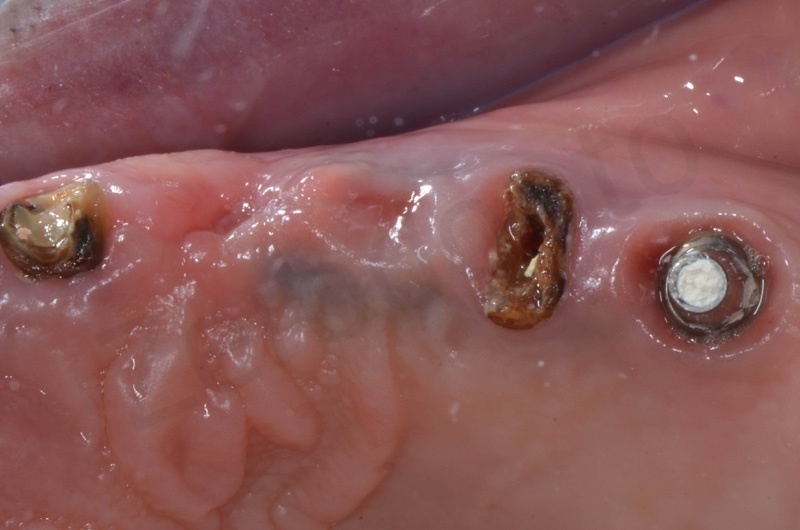
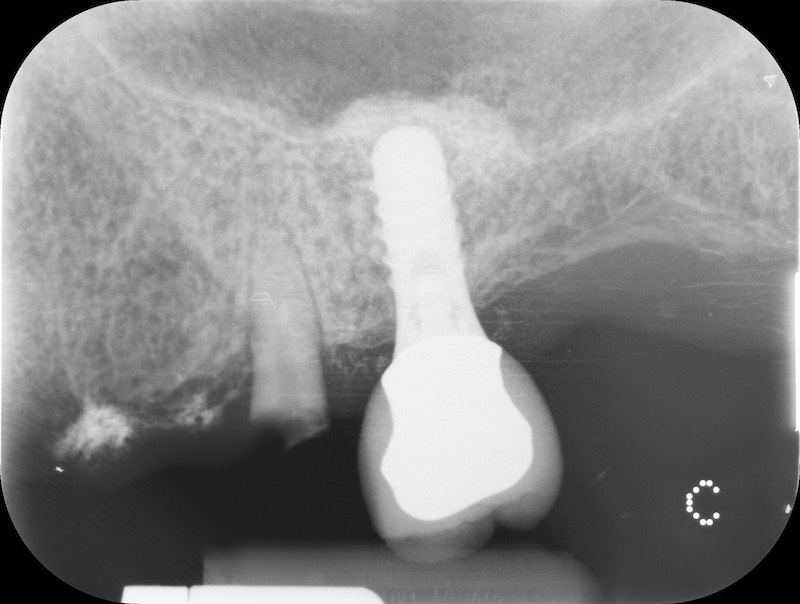
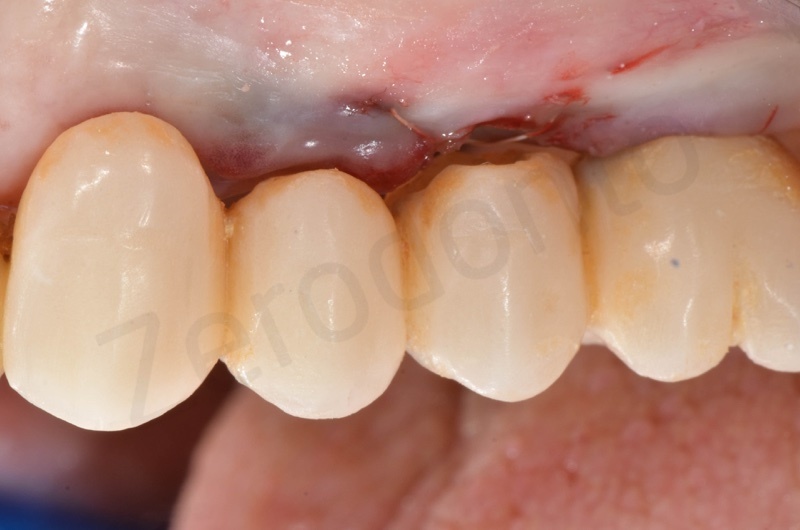
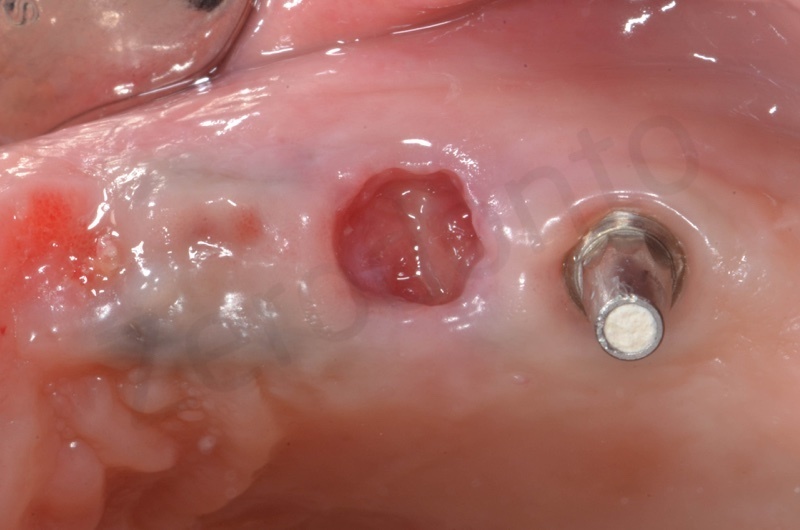
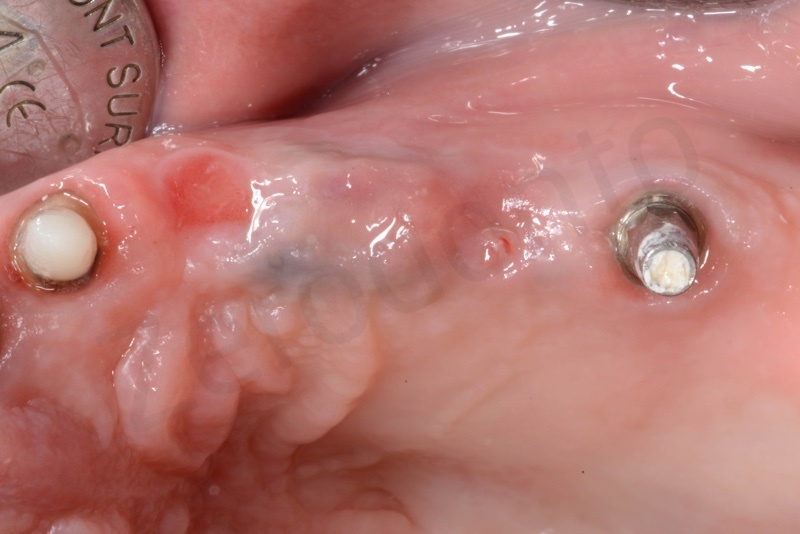
Bone healing after 3 months. The root of #25 is extracted and an all-on-four is placed.
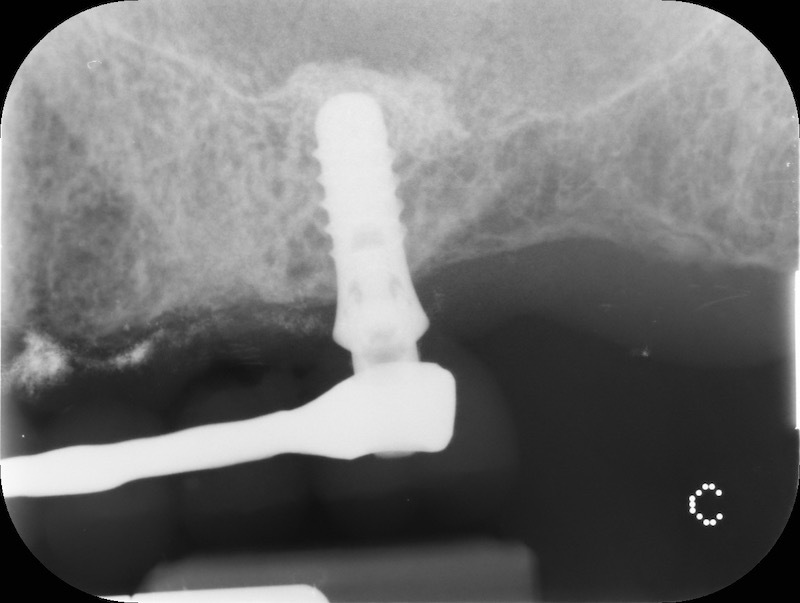
Video: Case report 3 – socket preservation in a case of buccal plate dehiscence
Bio-Oss® collagen + graft from the tuber region
Female patient, 43 yo. Severe gum disease.
Starting with a non surgical periodontal therapy, periodontal stabilization and orthodontic therapy with active retainers.


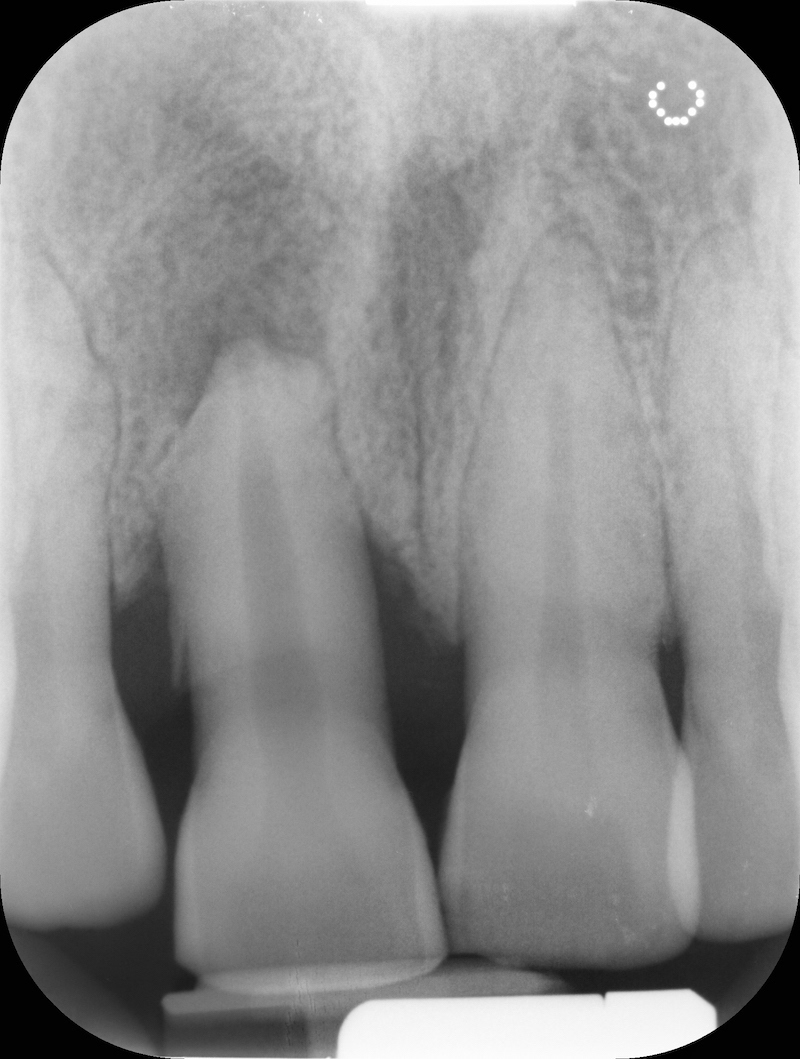
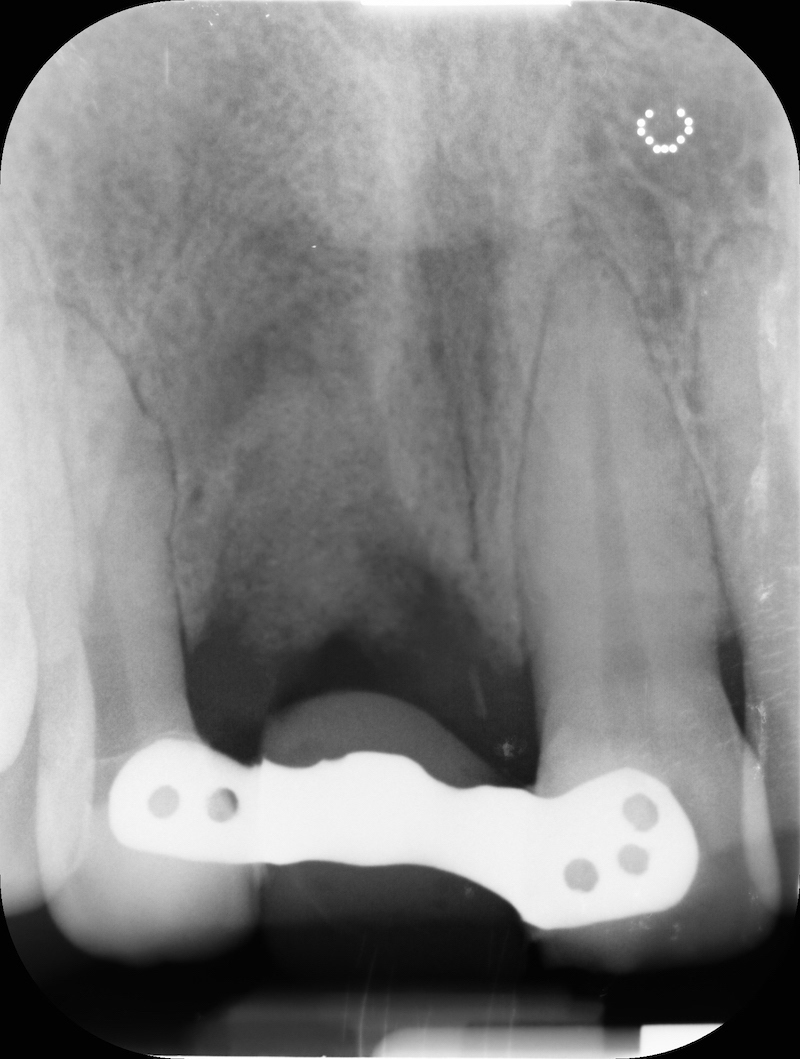
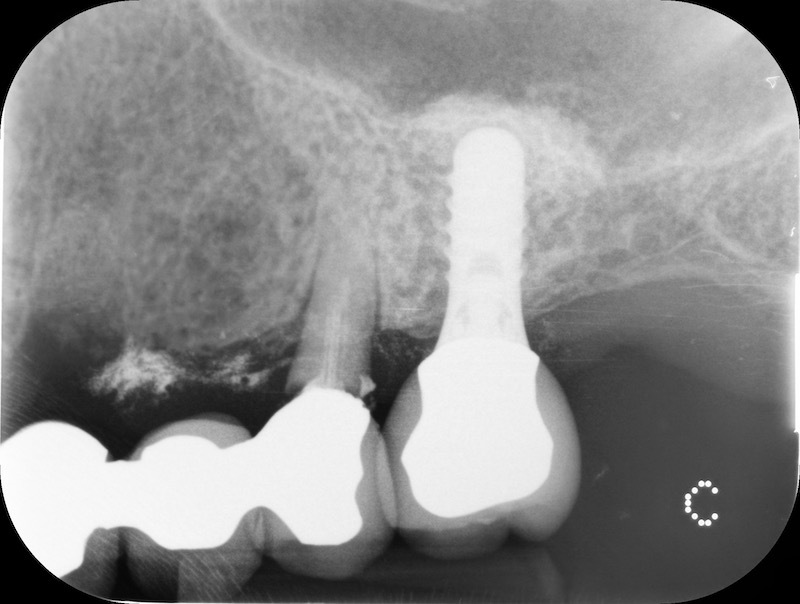
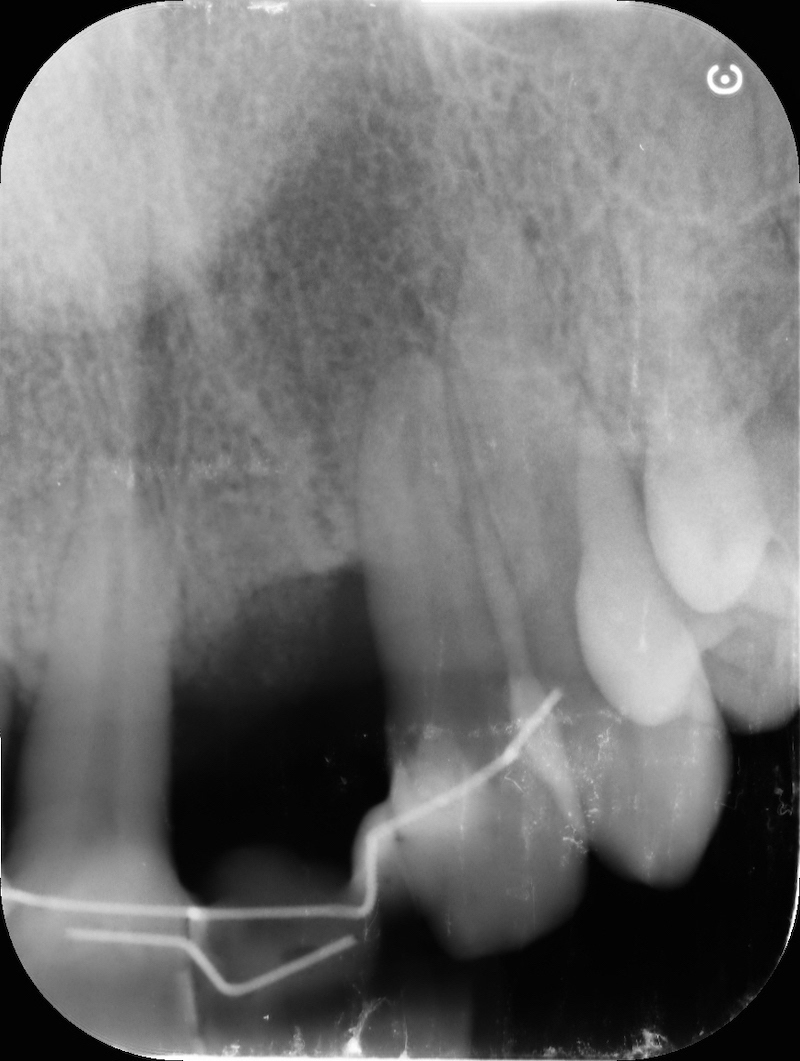
Pre-op RX.
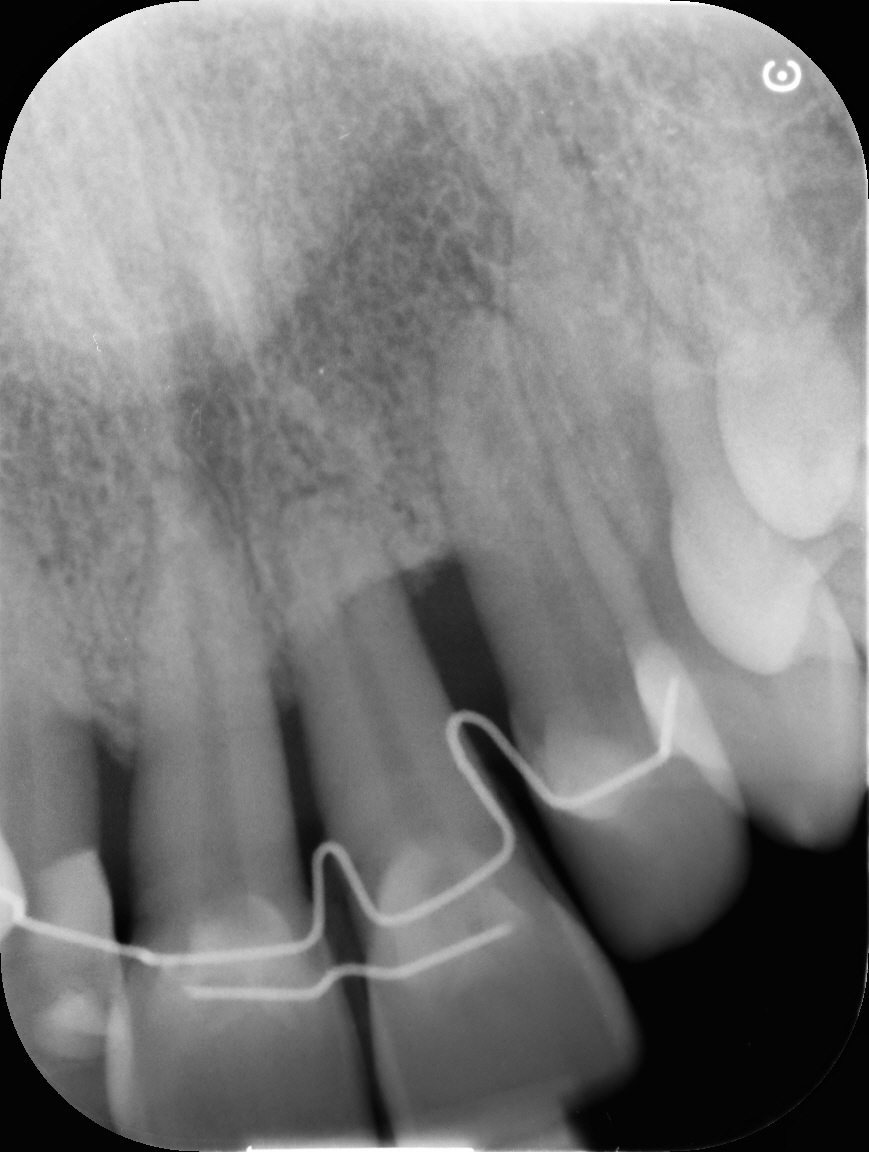
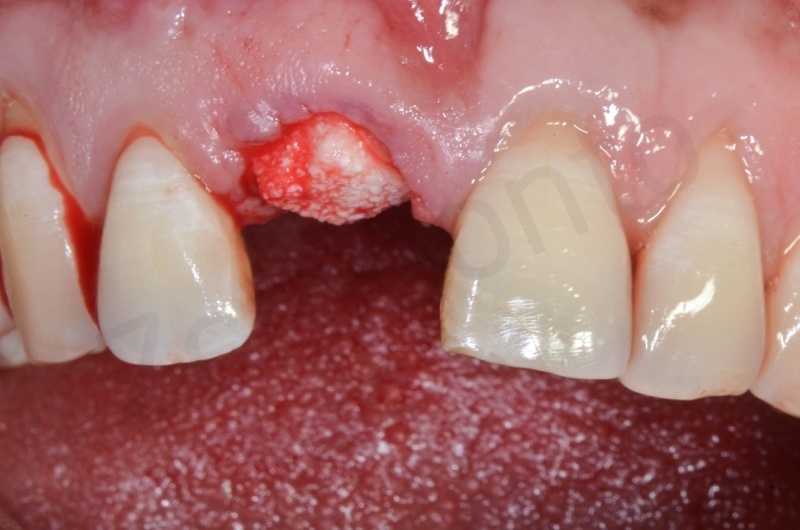
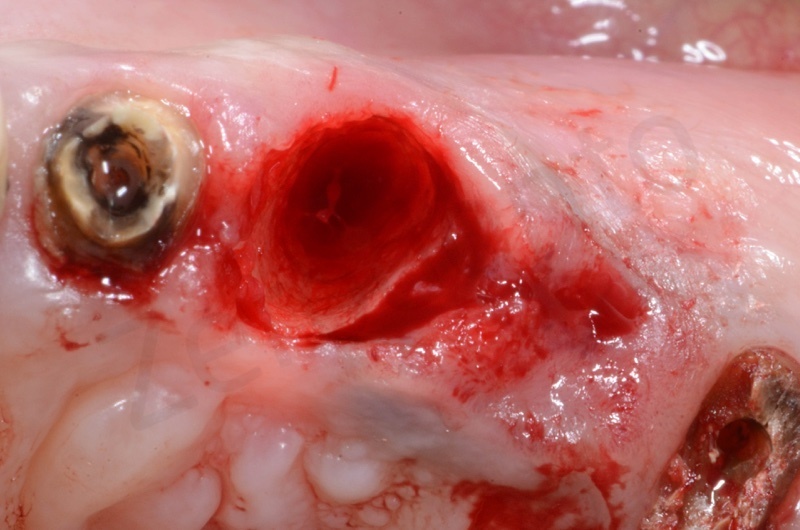
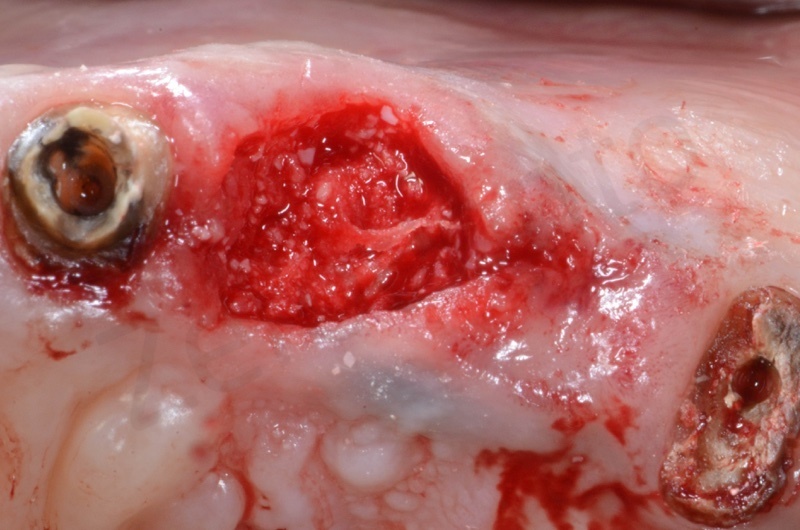
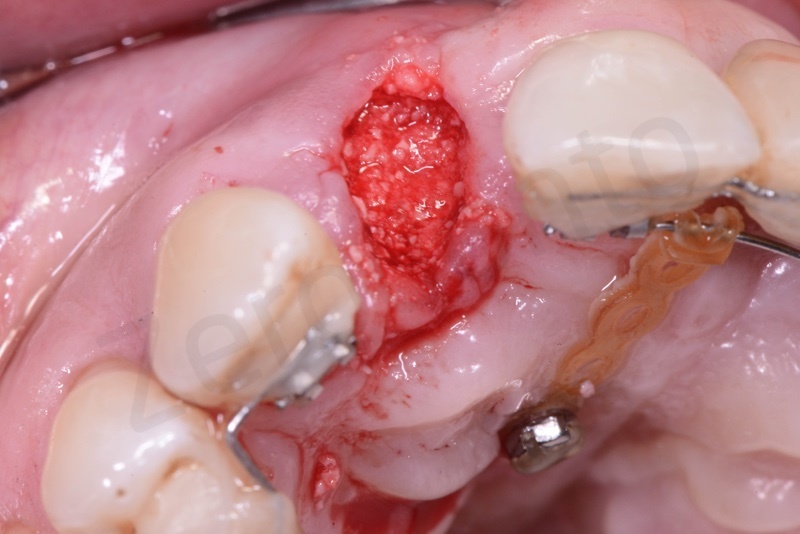
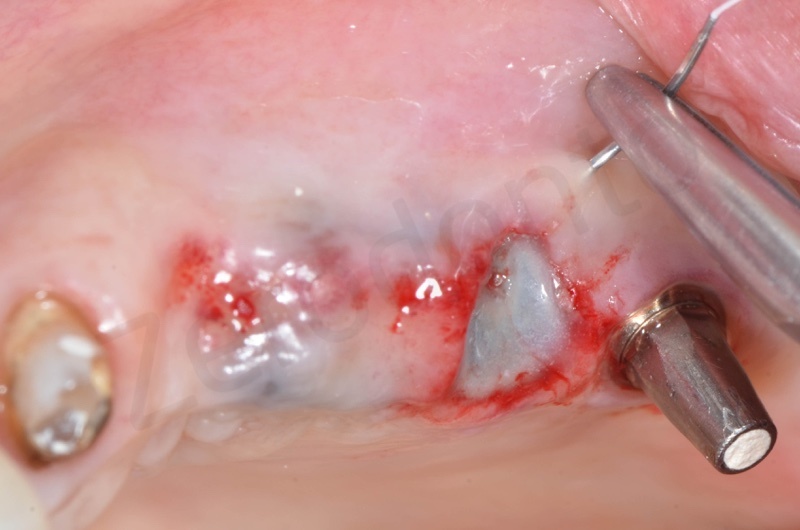
After avulsion, we carefully removed inflamed epithelium and granulation tissue around the border of the socket.




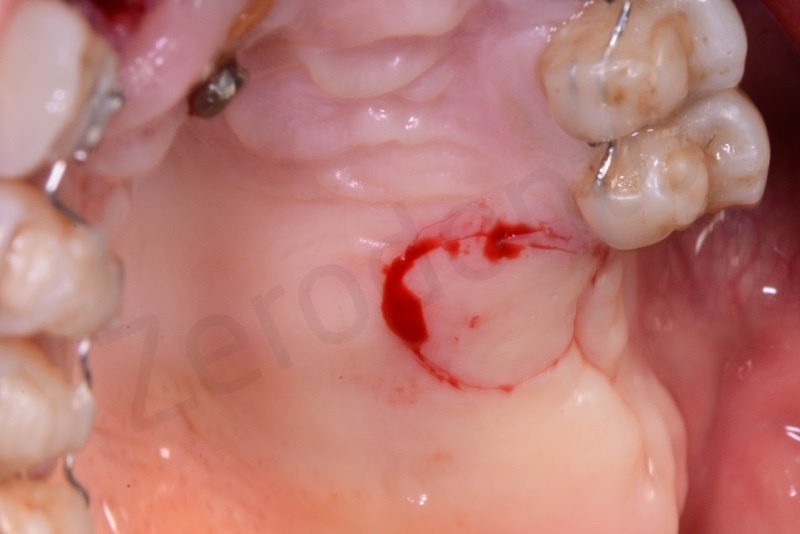
This site was chosen for connective tissue grafting from the tuber region.

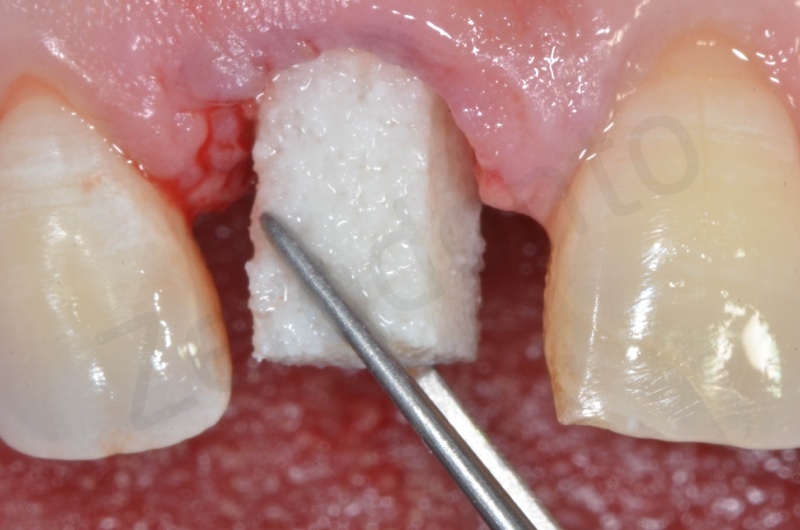
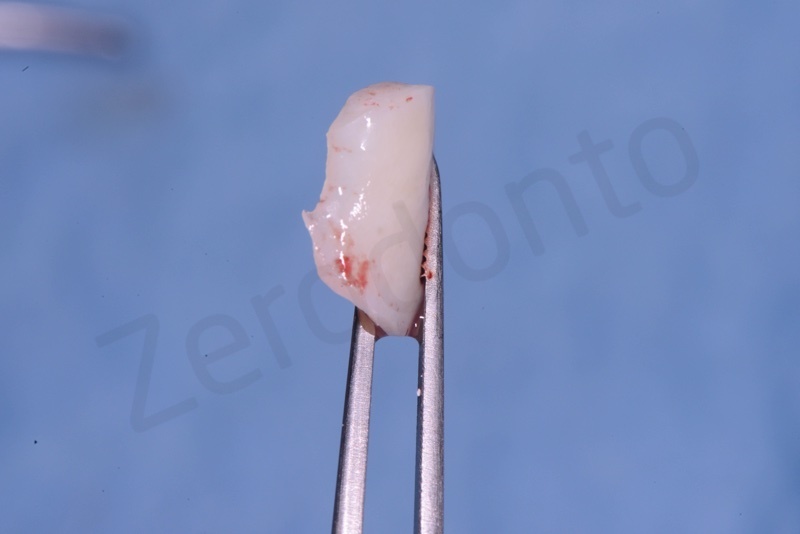
Bio-Oss® collagen appears as a rigid small block. It is hydrated with saline solution.

Bio-Oss collagen inserting in the alveolar socket

Material must be positioned at the level of bone margin.


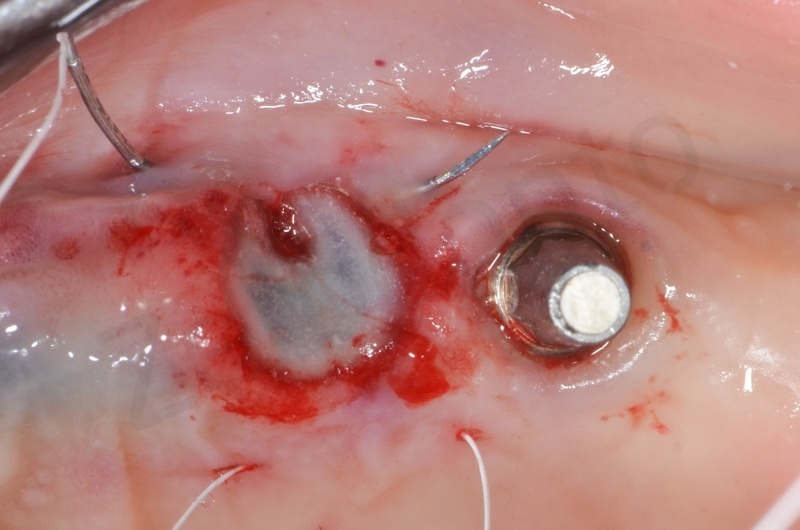
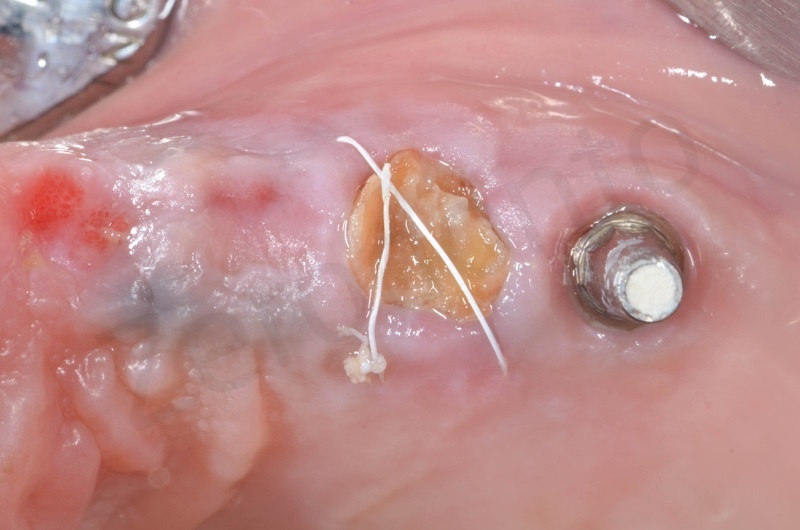
Connective tissue grafted from the tuber region.

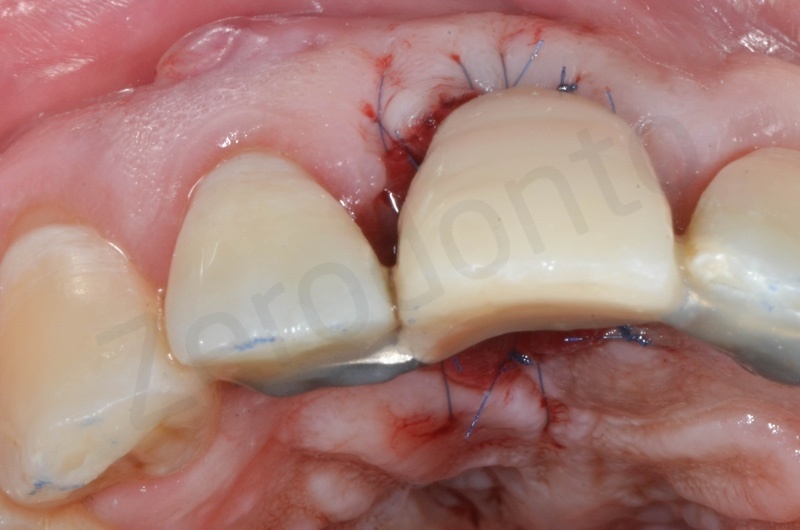
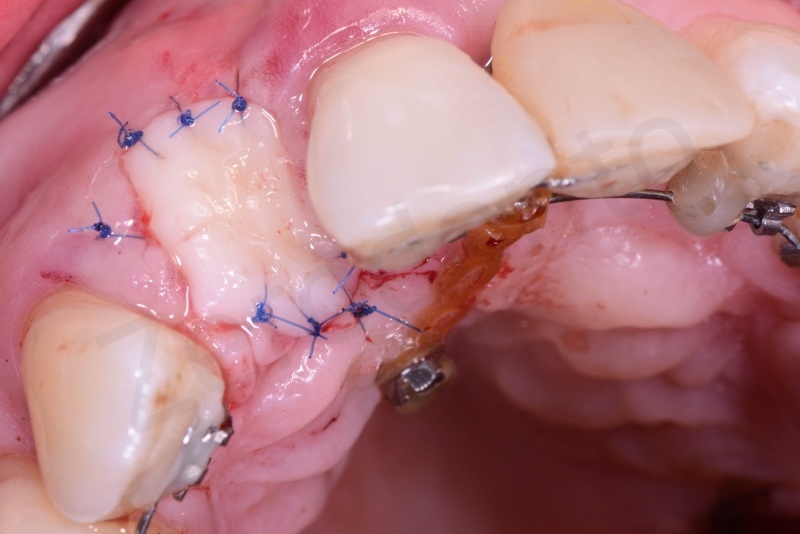
Tissue graft is anchored to the alveolar socket by means of 6.0 polypropylene suture.




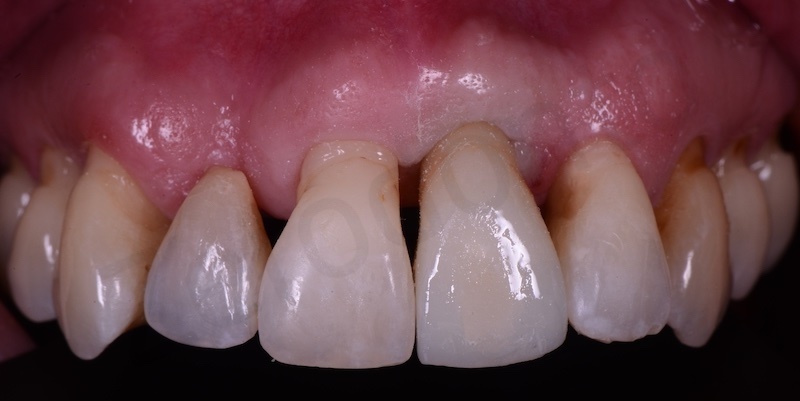
A maryland is positioned.

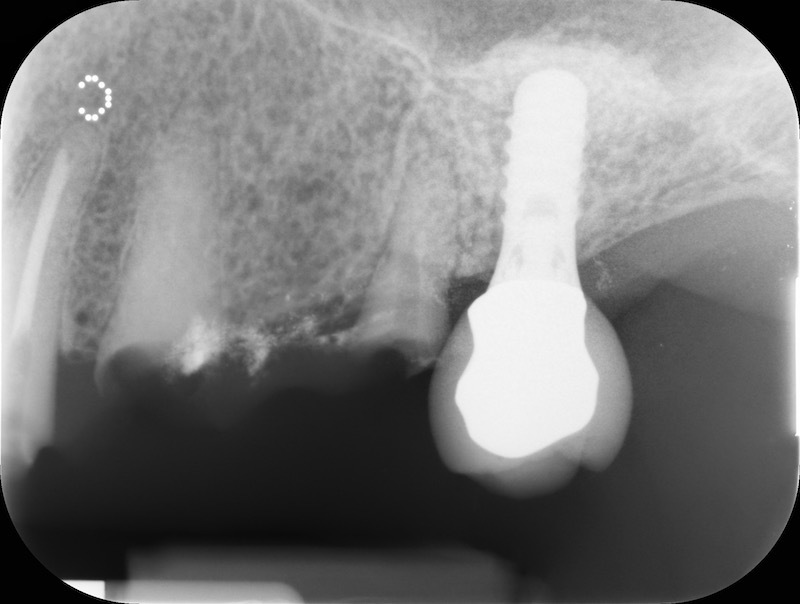
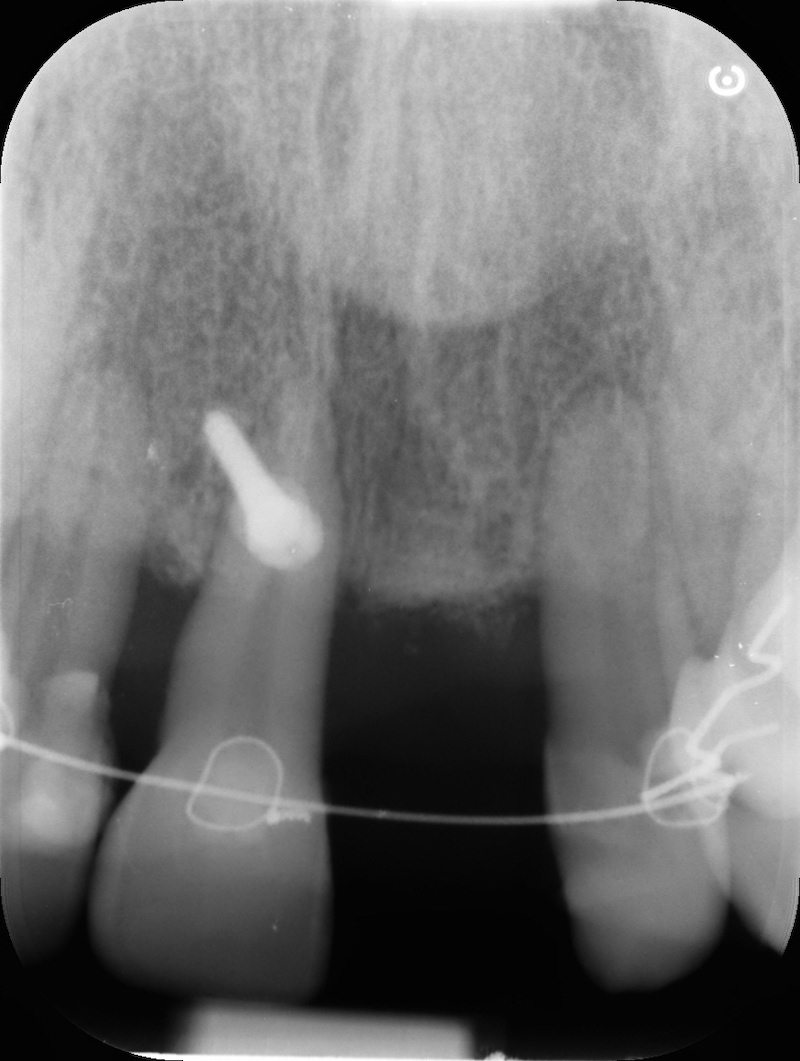
Post-op RX
1 week healing


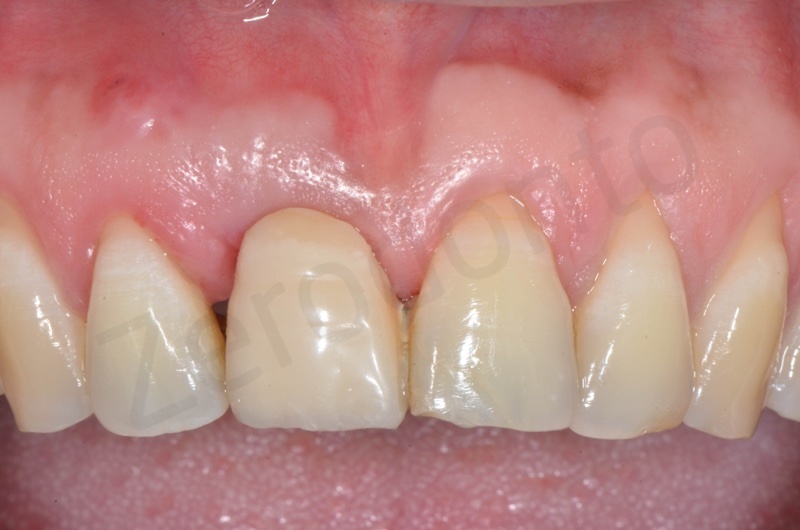
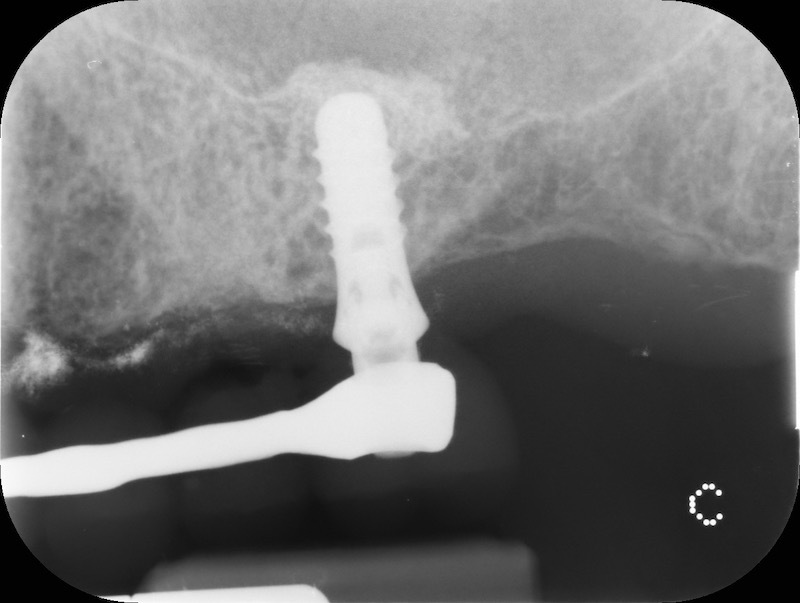
Rx after 13 months
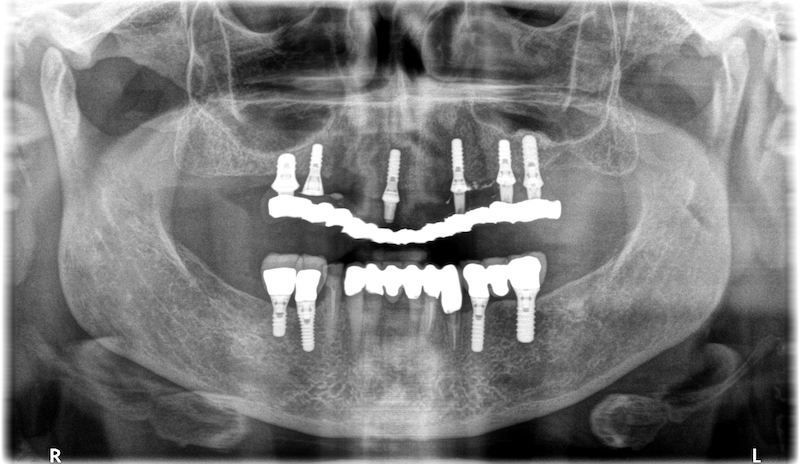
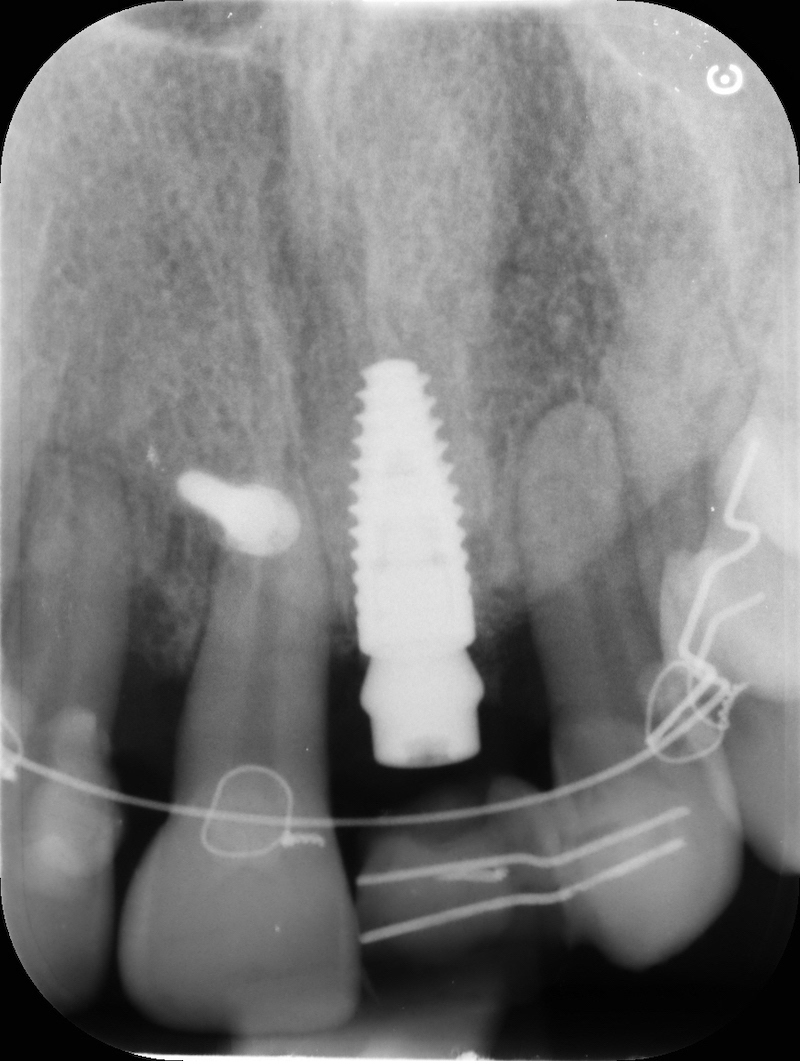
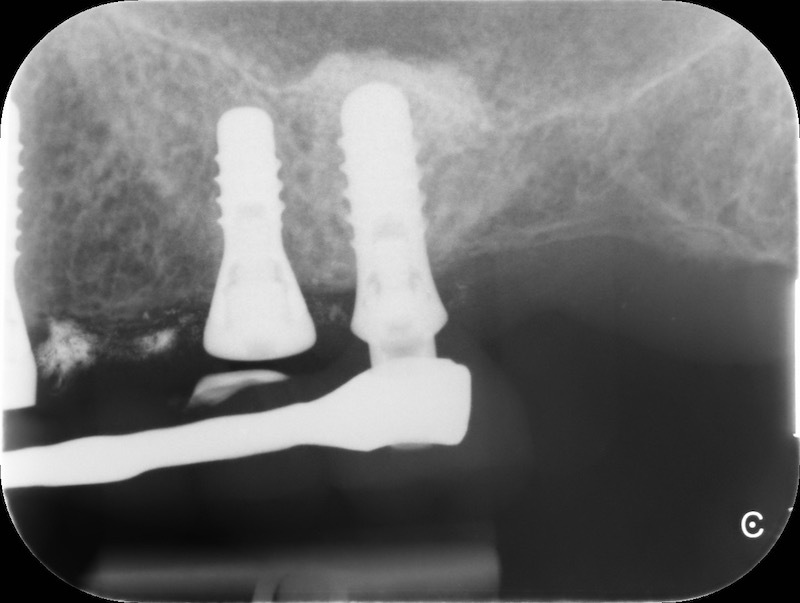
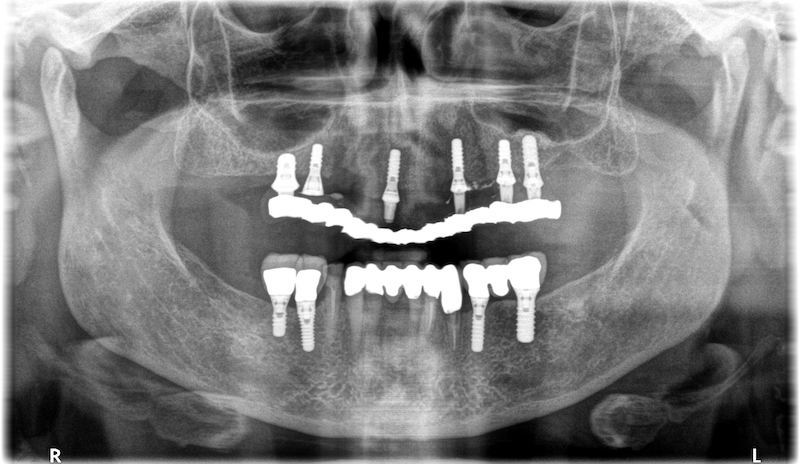
RX and implant insertion after 14 months
Bio-Oss® collagen + connective tissue graft from palate
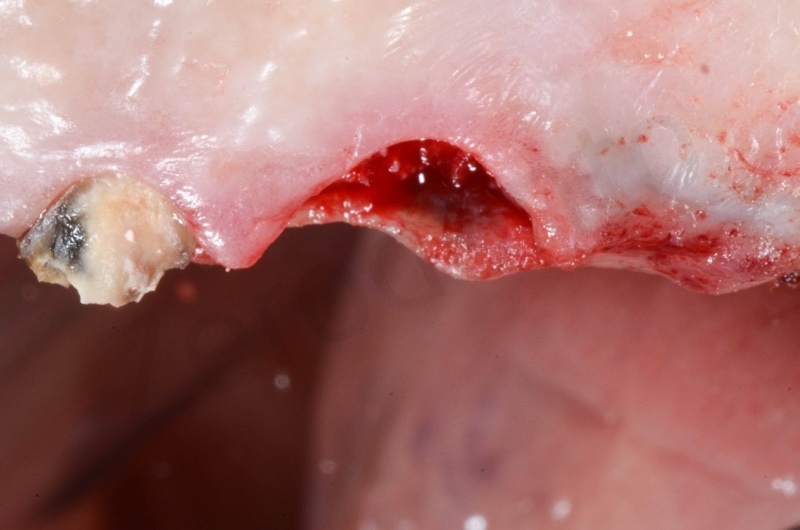
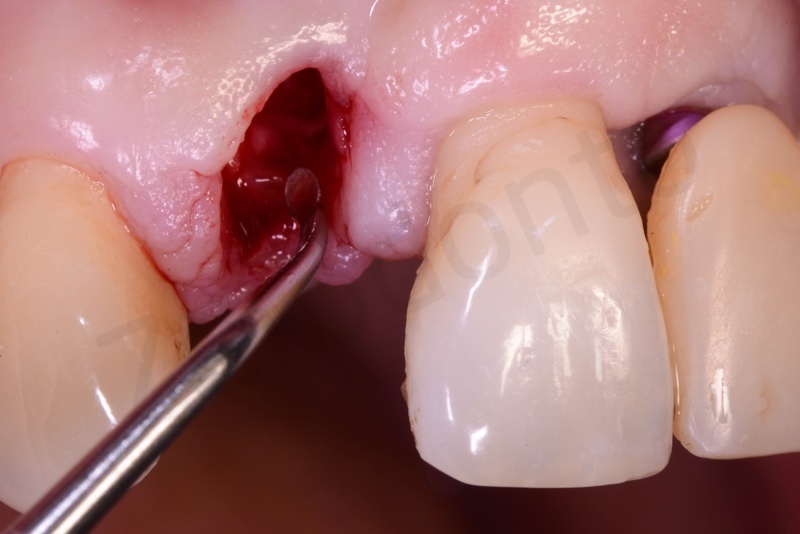
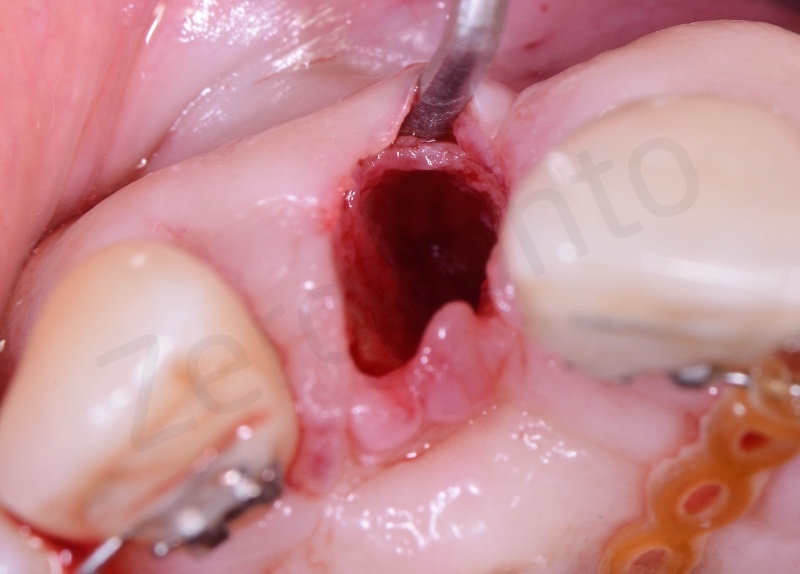
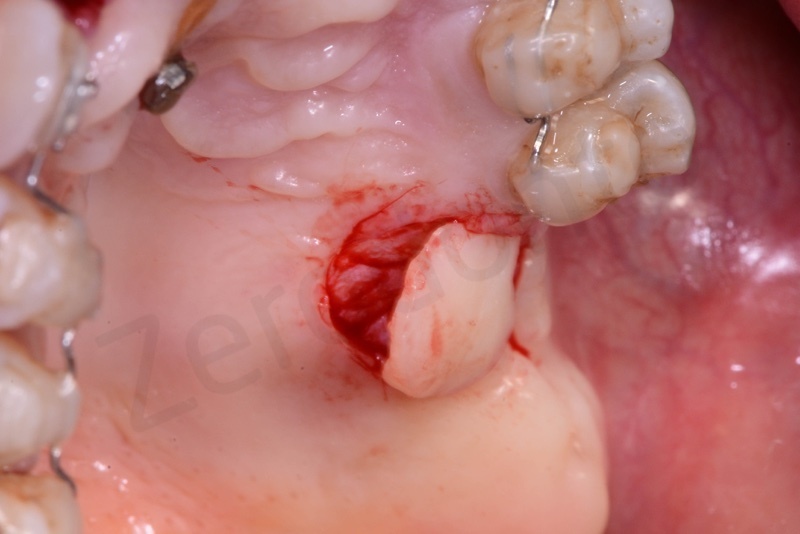
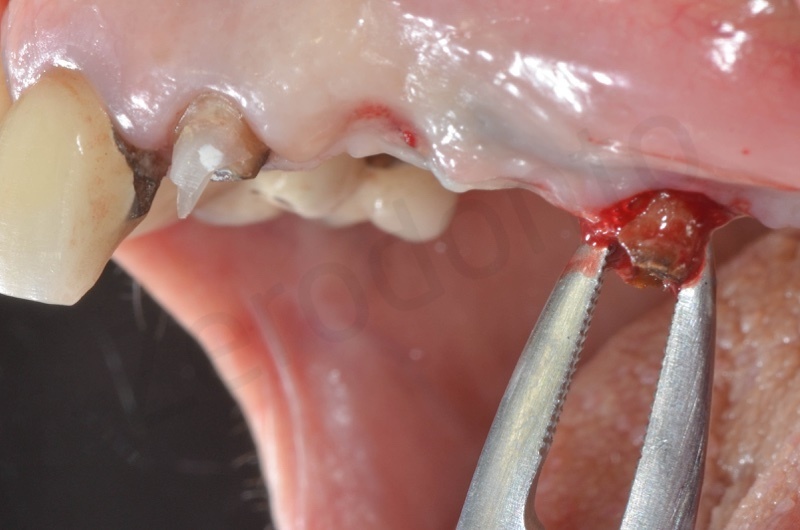
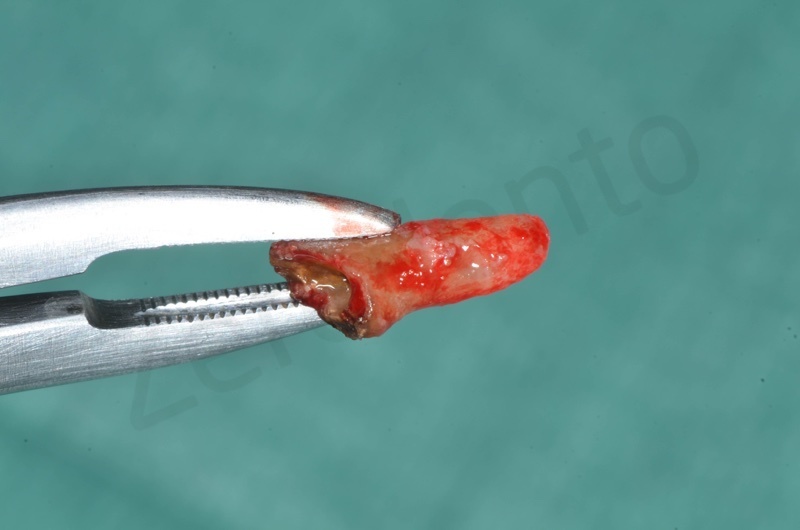
Atraumatic avulsion of #12 by means of a straight elevator.
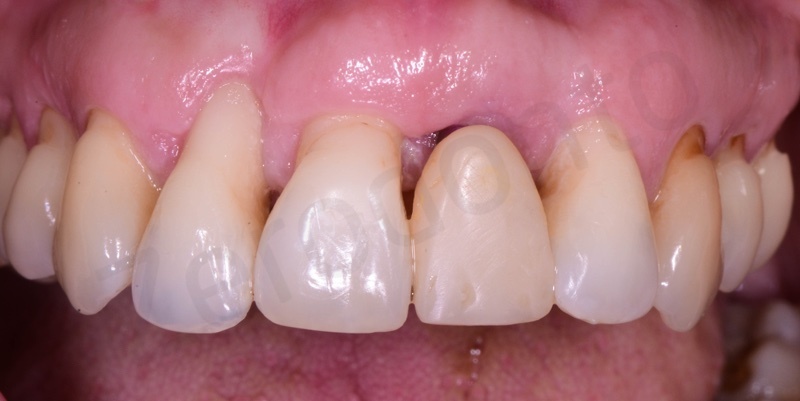
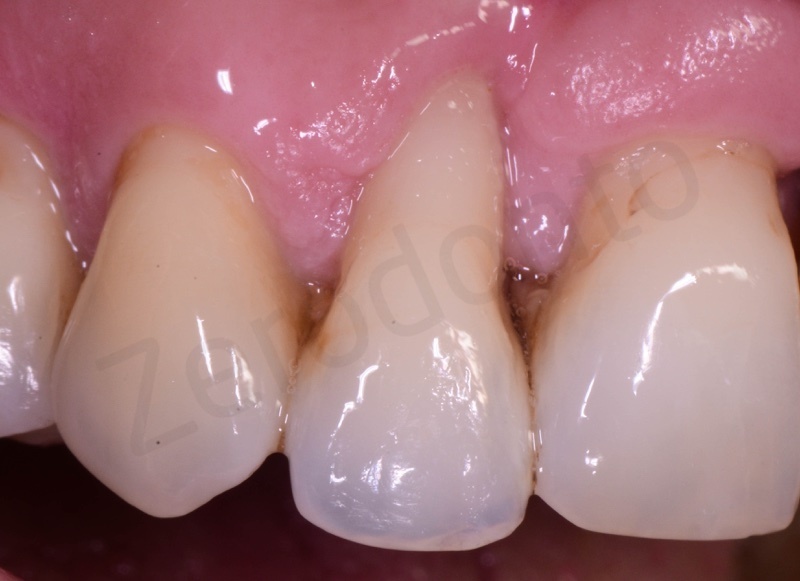
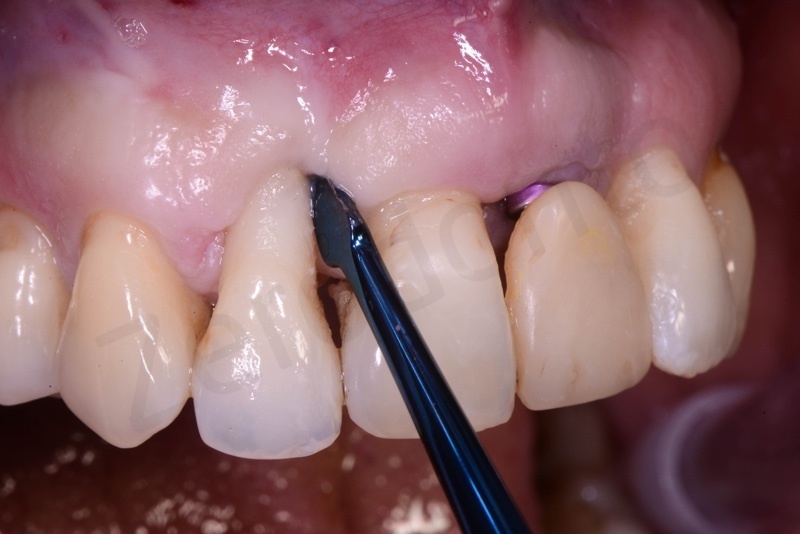
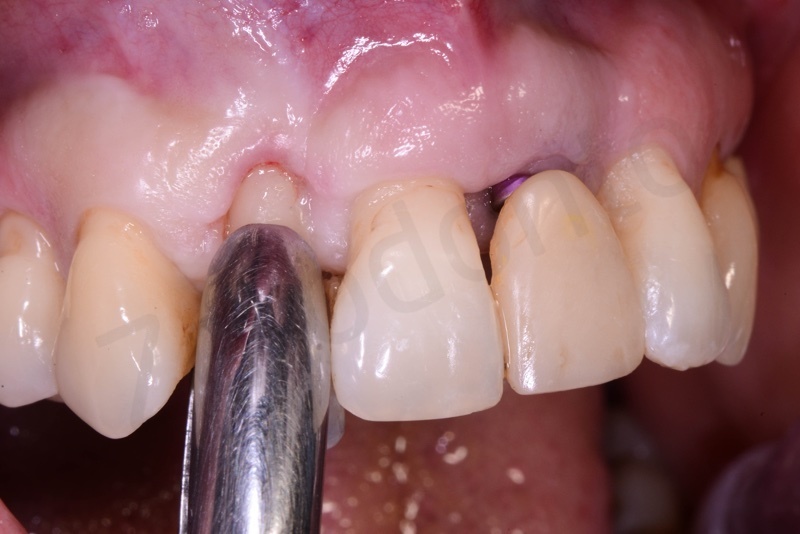
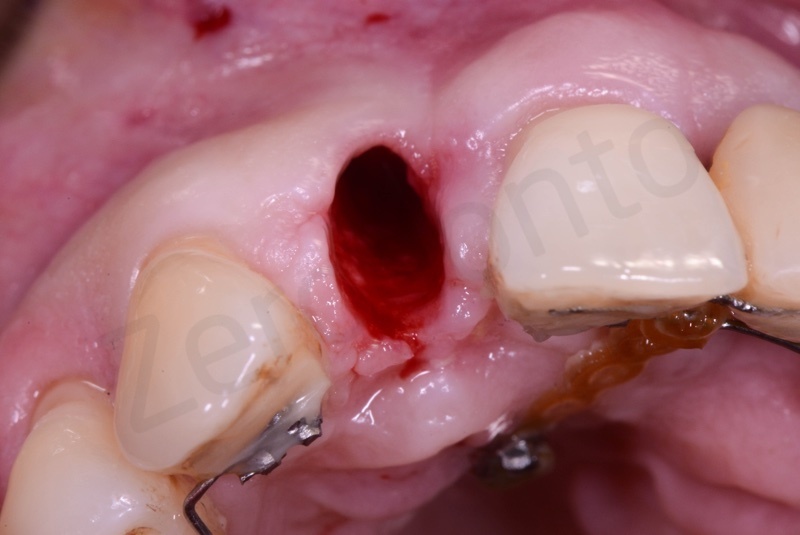
Curettage of granulation tissue.
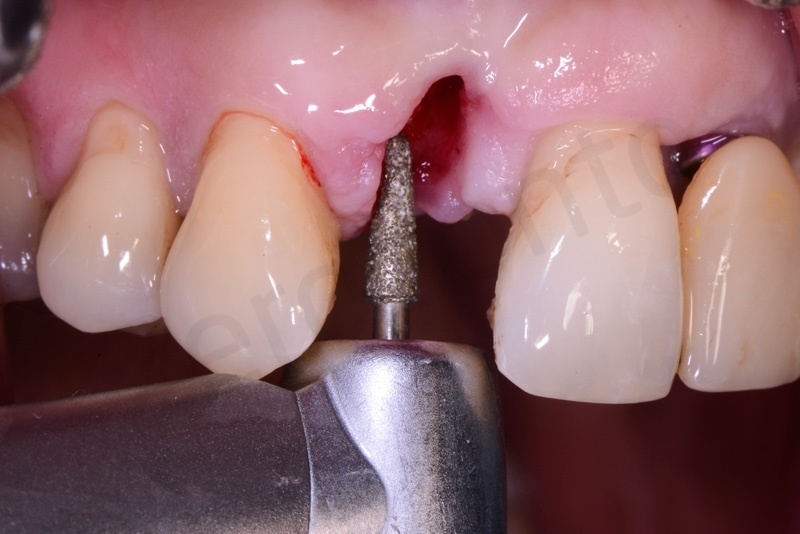
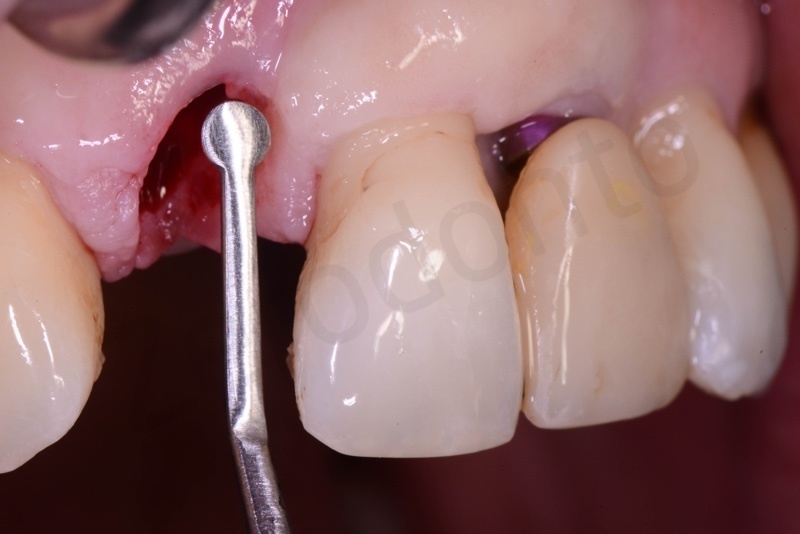
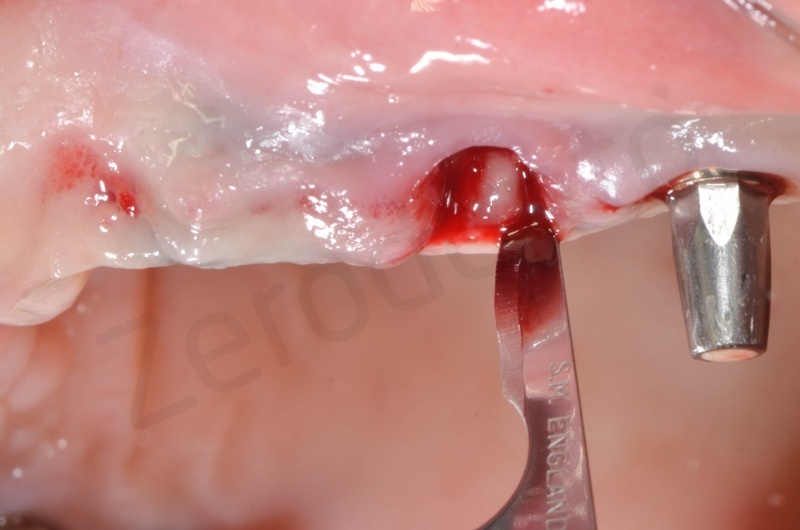
Connective tissue grafting from the palate.
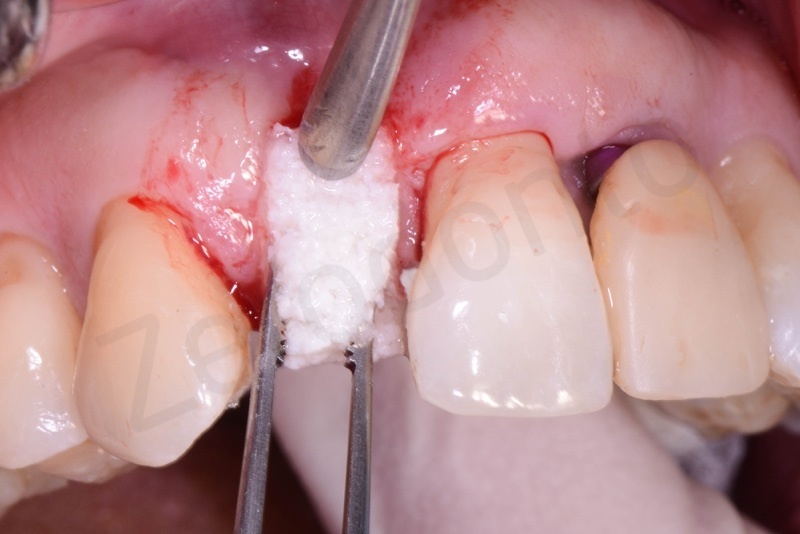
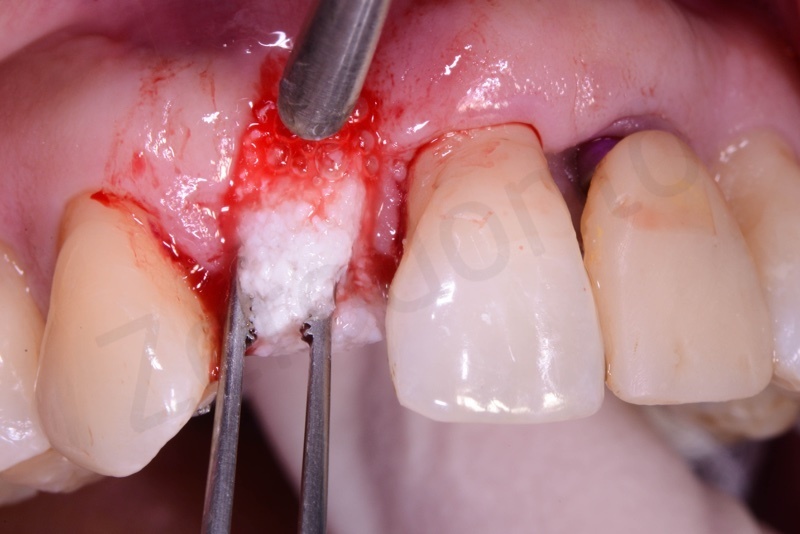
Bio-Oss® collagen application.
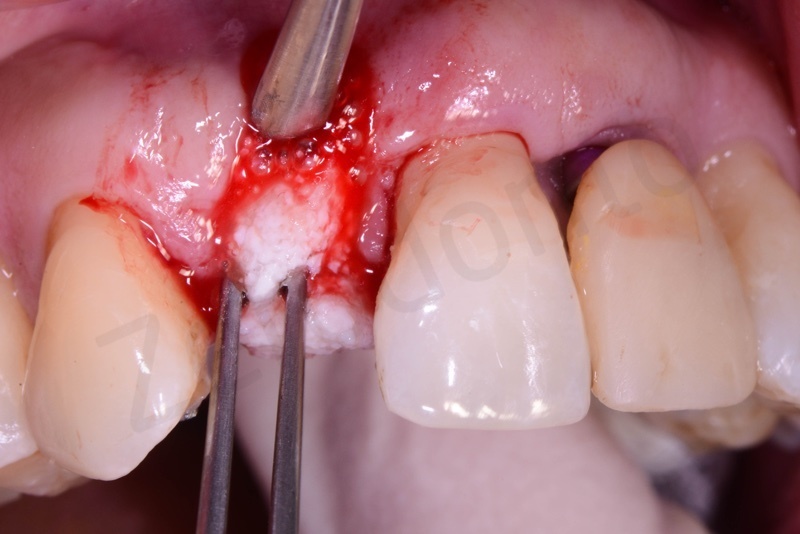
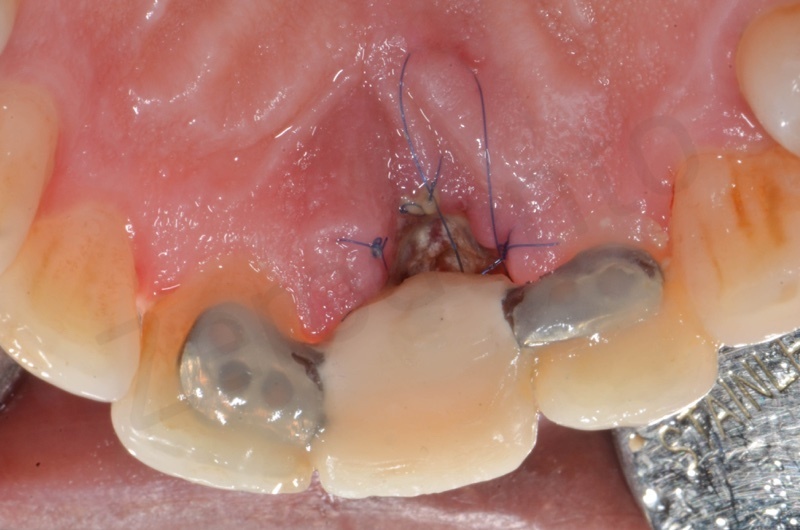
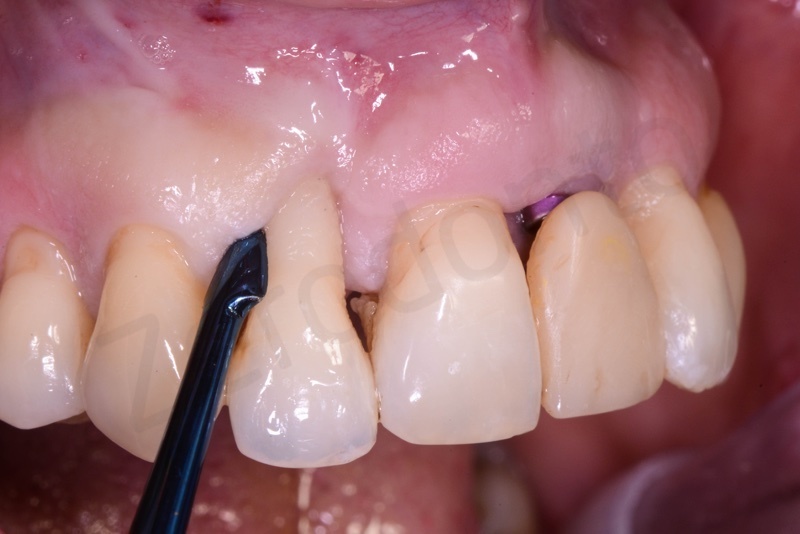
Application of connective tissue graft punch to the keratinized gingiva with sutures.
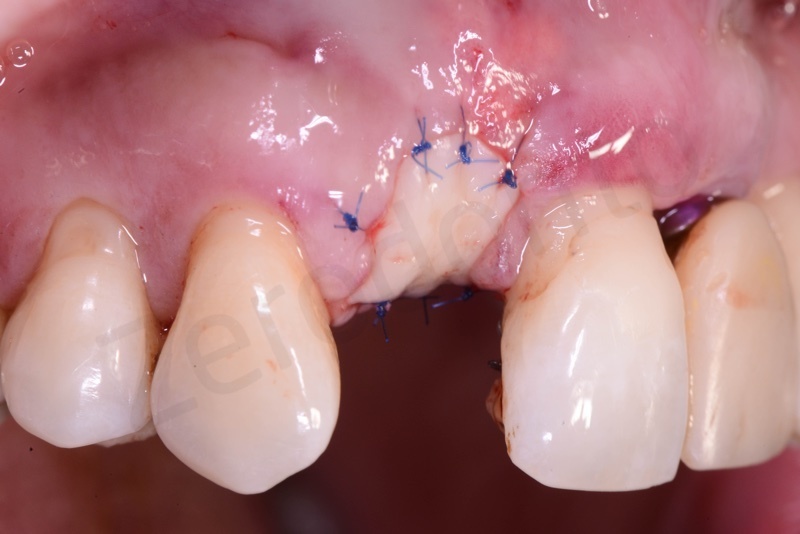
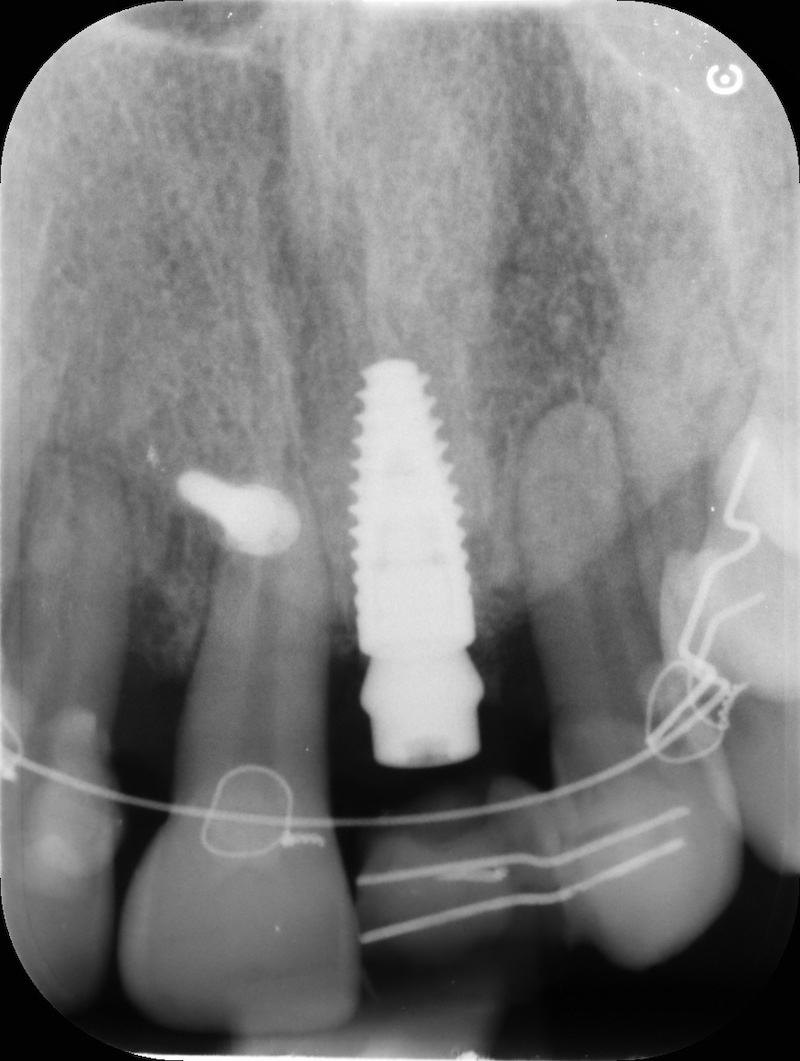
Implant insertion.
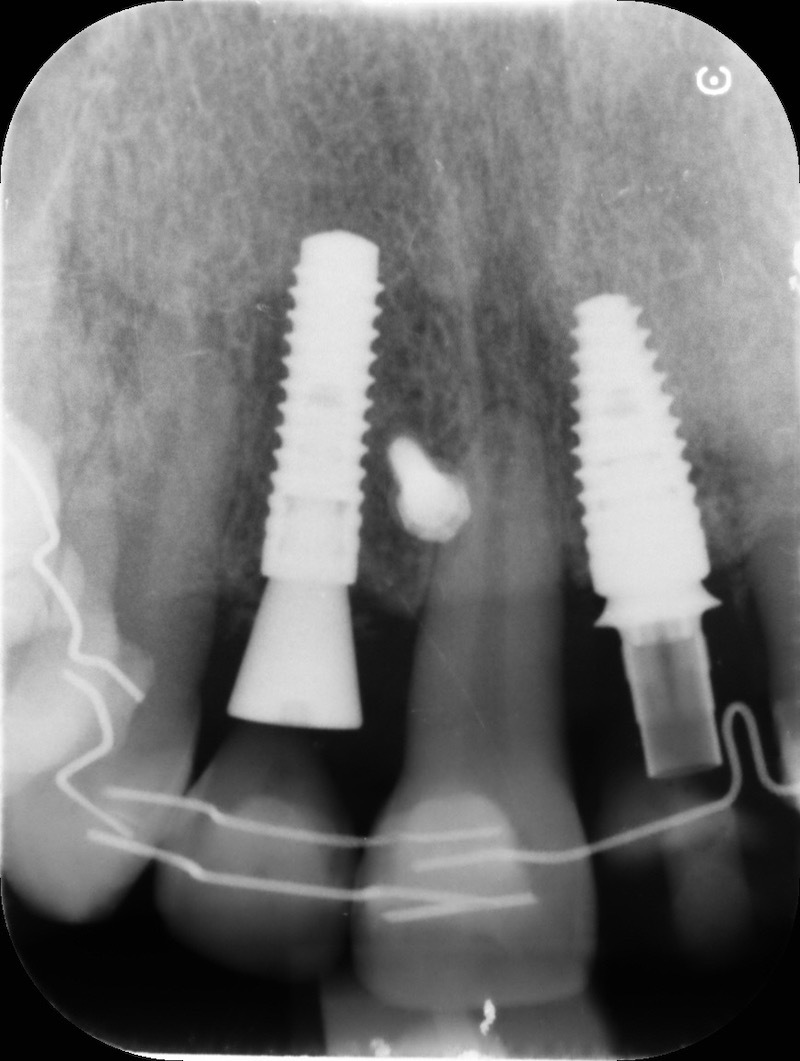
Bio-Oss® collagen + membragel
The Membragel is a gel membrane made of polyethylene glycol that has to be applied with a special dispenser for direct mixing of two synthetic components, which solidifies in a little less than a minute stabilizing the granules of biomaterial in their most superficial layer.
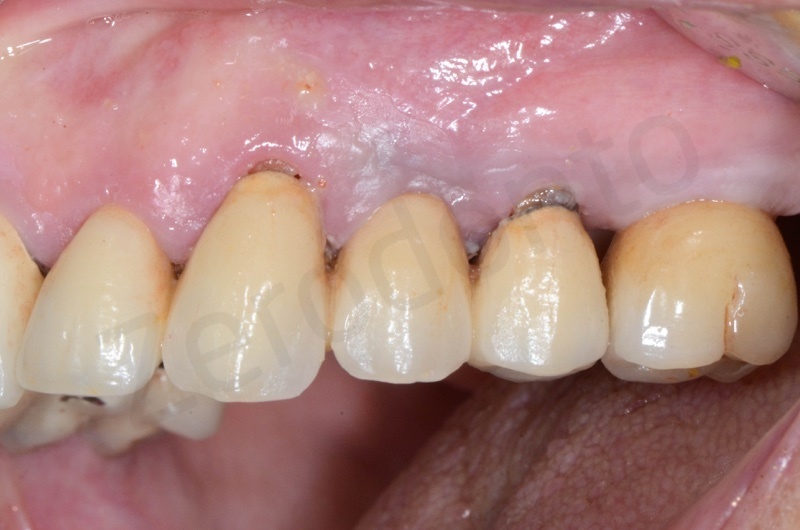
In the patient who previously had a socket in region 23 (case number two, upper in this article), three months after the surgery, we are also preparing to extract the root of 25, with ridge preservation in view of future implant placement.
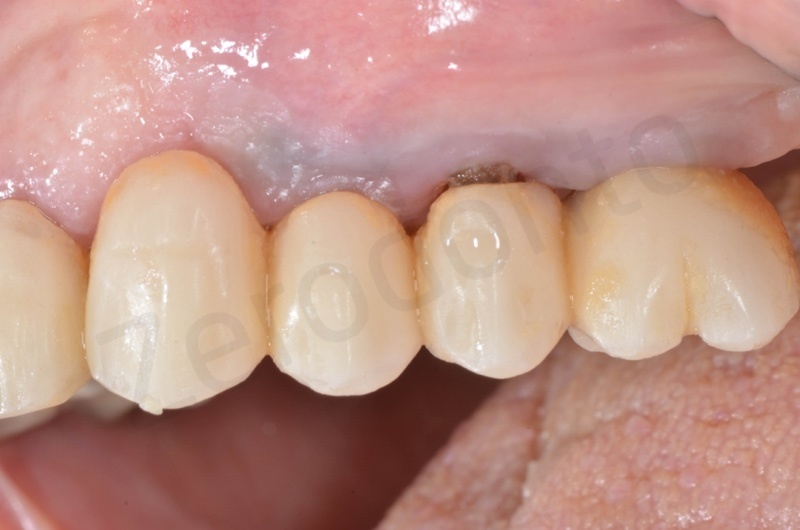
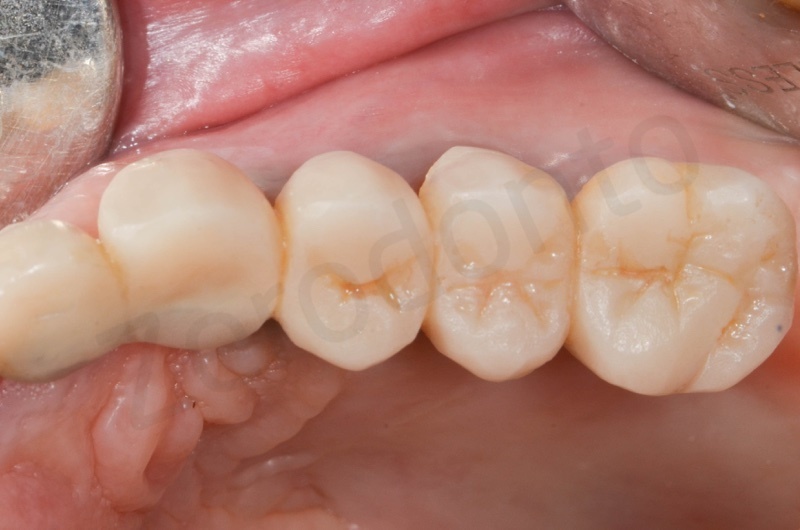
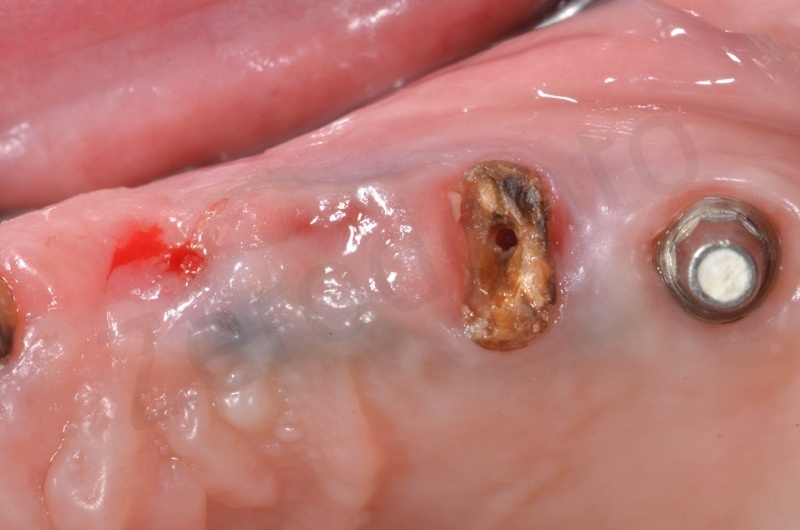
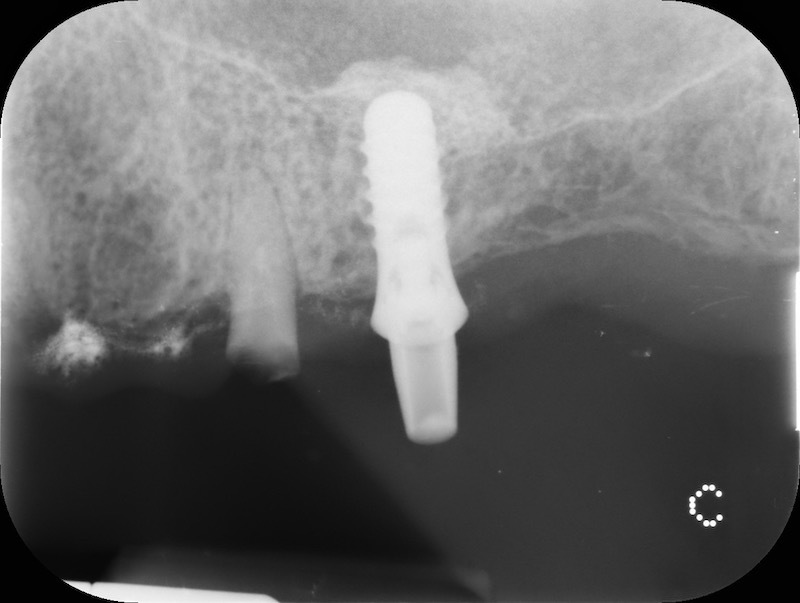
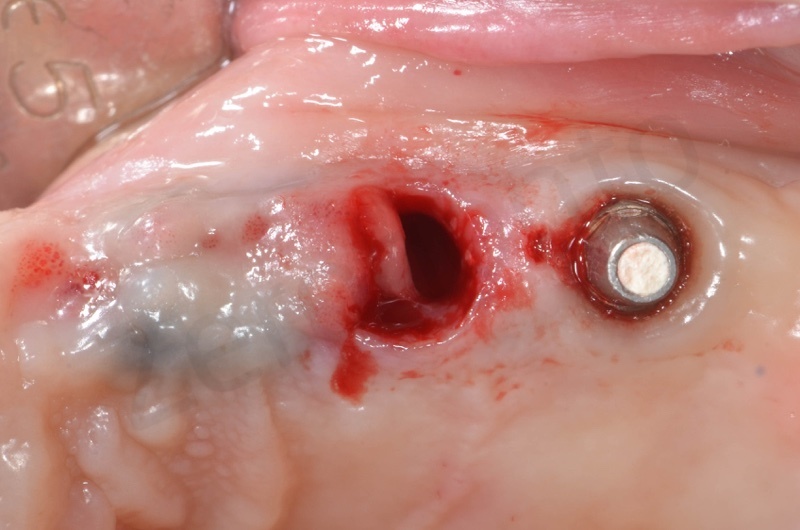
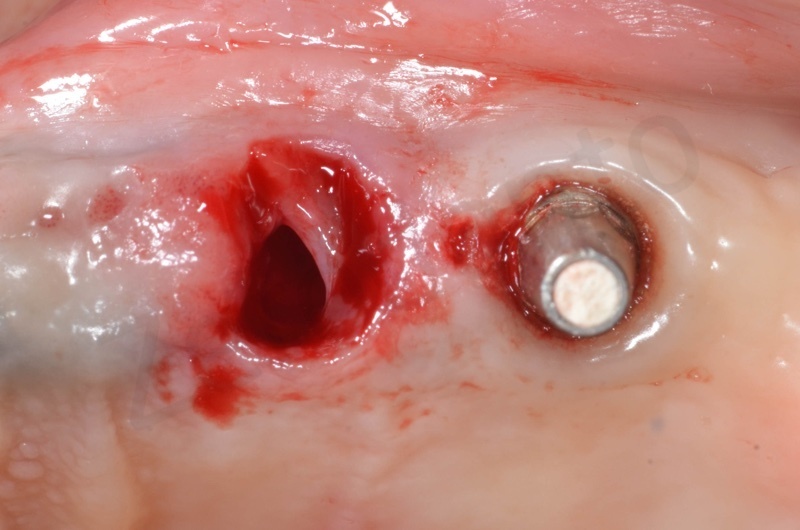
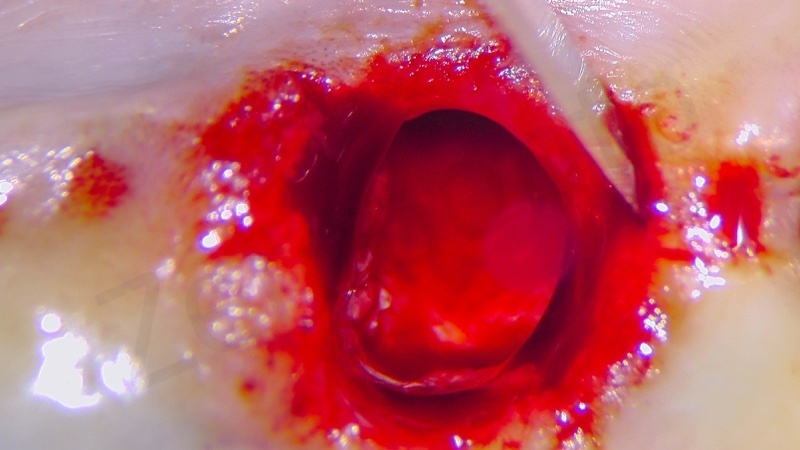
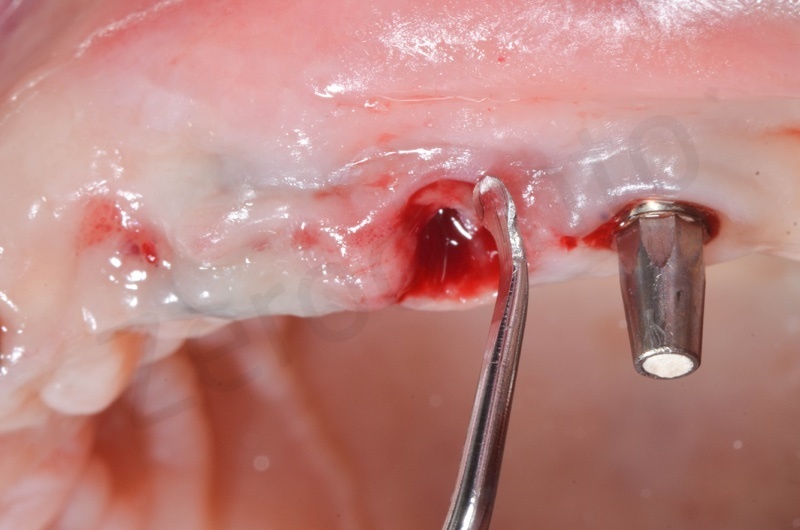
CONCLUSIONS
To date, no technique has yet succeeded in preserving the ridge completely and we do not yet have the ideal material for the socket. However, the socket preservation is able to limit bone resorption.
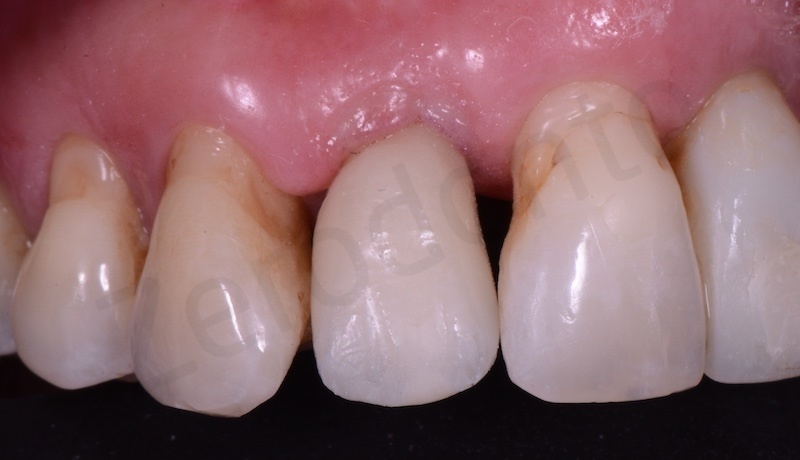
From a clinical point of view, according to our experience, the use of the connective graft results in better healing of the tissues in the weeks following the intervention. Membragel, on the other hand, from a clinical point of view, is the material that seems to us less performing. The use of Mucograft seemed to us the best solution considering the fact that the removal of connective tissue is avoided, thus reducing the patient’s morbidity.
REFERENCES
Araujo, M.G. & Lindhe, J. (2005) Dimensional ridge alterations following tooth extraction: an experi- mental study in the dog. Journal of Clinical Peri- odontology 32: 212–218.
Atwood, D. A. (1962) Some clinical factors related to the rate of resorption of residual ridges. Journal of Prosthetic Dentistry 12, 441–450.
Cardaropoli, G., Arau ́jo, M. & Lindhe, J. (2003) Dynamics of bone tissue formation in tooth extraction sites. An experimental study in dogs. Journal of Clinical Periodontology 30, 809–818.
Vignoletti, F., Matesanz, P., Rodrigo, D., Figuero, E., Martin, C. & Sanz, M. (2012) Surgical proto- cols for ridge preservation after tooth extraction. A systematic review. Clinical Oral Implants Research 23(Suppl. 5): 22–38.
Barone, A., Orlando, B., Cingano, L., Marconcini, S., Derchi, G. & Covani, U. (2012) A randomised clinical trial to evaluate and compare implants placed in augmented vs. non-augmented extrac- tion sockets: a 3-year evaluation. Journal of Peri- odontology 83: 836–846.
Barone, A., Ricci, M., Tonelli, P., Santini, S. & Covani, U. (2013) Tissue changes of extraction sockets in humans. A comparison of spontaneous healing vs. ridge preservation with secondary soft tissue healing. Clinical Oral Implants Research 24: 1231–1237.
Barone, A., Toti, P., Piattelli, A., Iezzi, G., Derchi, G. & Covani, U. (2014) Extraction socket healing in humans after ridge preservation techniques: a comparison between flapless and flapped proce- dure in a randomised clinical trial. Journal of Periodontology 85: 14–23.
Cardaropoli, D. & Cardaropoli, G. (2008) Preserva- tion of the postextraction alveolar ridge. A clini- cal and histologic study. The International Journal of Periodontics & Restorative Dentistry 28: 469–477.
Fickl, S., Zuhr, O., Wachtel Bolz, W. & Hu€rzeler, M.B. (2008b) Tissue alteration after tooth extrac- tion with and without surgical trauma: a volu- metric study in beagle dog. Journal of Clinical Periodontology 35: 356–363.
Fickl, S., Zuhr, O., Wachtel, H., Stappert, C.F.J., Stein, J.M. & Hu€rzeler, M.B. (2008a) Dimensional changes of the alveolar ridge contour after differ- ent socket preservation techniques. Journal of Clinical Periodontology 35: 906–913.
Schropp, L., Wenzel, A., Kostopoulos, L. & Karring, T. (2003) Bone healing and soft tissue contour changes following single-tooth extraction: a clini- cal and radiographic 12-month prospective study. The International Journal of Periodontics & Restorative Dentistry 23: 313–323.
Schropp, L., Kostopoulos, L., Wenzel, A. & Isidor, F. (2005) Clinical and radiographic performance of delayed-immediate single- tooth implant placement associated with peri-implant bone defects. A 2-year prospec- tive, controlled, randomized follow-up report. Journal of Clinical Periodontology 32, 480– 487.
Arau ́ jo, M., Linder, E., Wennstro ̈ m, J. & Lindhe, J. (2008) The influence of Bio-Oss Collagen on healing of an extraction socket: an experimental study in the dog. Interna- tional Journal of Periodontics and Restora- tive Dentistry 28, 123–135.
Jung, R. E., Siegenthaler, D. W. & Hammerle, C. H. (2004) Postextraction tissue manage- ment: a soft tissue punch technique. Interna- tional Journal of Periodontics and Restorative Dentistry 24, 545–553.
Carmagnola, D., Adriaens, P. & Berglundh, T. (2003) Healing of human extraction sockets filled with Bio-Oss. Clinical Oral Implants Research 14, 137–143.
Cardaropoli, M. Arau ́ jo, R. Hayacibara, F. Sukekava, and J. Lindhe, “Healing of extraction sockets and surgically pro- duced—augmented and non-augmented—defects in the al- veolar ridge. An experimental study in the dog,” Journal of Clinical Periodontology, vol. 32, no. 5, pp. 435–440, 2005.
C. H. Hammerle, M. G. Araujo, and M. Simion, “Evidence- based knowledge on the biology and treatment of extraction sockets,” Clinical Oral Implants Research, vol. 23, supplement 5, pp. 80–82, 2012.
M. Araùjo, E. Linder, and J. Lindhe, “Effect of a xenograft on early bone formation in extraction sockets: an experimental study in dog,” Clinical Oral Implants Research, vol. 20, no. 1, pp. 1–6, 2009.
S.Fickl, D.Schneider,O.Zuhretal.,“Dimensionalchangesof the ridge contour after socket preservation and buccal over- building: an animal study,” Journal of Clinical Periodontology, vol. 36, no. 5, pp. 442–448, 2009.
D. Botticelli, T. Berglundh, D. Buser, and J. Lindhe, “The jumping distance revisited: an experimental study in the dog,” Clinical Oral Implants Research, vol. 14, no. 1, pp. 35– 42, 2003.
D. Botticelli, T. Berglundh, and J. Lindhe, “Resolution of bone defects of varying dimension and configuration in the marginal portion of the peri-implant bone: an experimental study in the dog,” Journal of Clinical Periodontology, vol. 31, no. 4, pp. 309–317, 2004.
M.G.Arau ́jo,J.L.Wennstro ̈m,andJ.Lindhe,“Modeling of the buccal and lingual bone walls of fresh extraction sites following implant installation,” Clinical Oral Implants Research, vol. 17, no. 6, pp. 606–614, 2006.
D. Botticelli, L. G. Persson, J. Lindhe, and T. Berglundh, “Bone tissue formation adjacent to implants placed in fresh extraction sockets: an experimental study in dogs,” Clinical Oral Implants Research, vol. 17, no. 4, pp. 351–358, 2006.
G. Cardaropoli, M. Arau ́ jo, R. Hayacibara, F. Sukekava, and
J. Lindhe, “Healing of extraction sockets and surgically pro- duced—augmented and non-augmented—defects in the al- veolar ridge. An experimental study in the dog,” Journal of Clinical Periodontology, vol. 32, no. 5, pp. 435–440, 2005.
C. H. Hammerle, M. G. Araujo, and M. Simion, “Evidence- based knowledge on the biology and treatment of extraction sockets,” Clinical Oral Implants Research, vol. 23, supplement 5, pp. 80–82, 2012.
F. Vignoletti, P. Matesanz, D. Rodrigo, E. Figuero, C. Martin, and M. Sanz, “Surgical protocols for ridge preservation after tooth extraction. A systematic review,” Clinical Oral Implants Research, vol. 23, supplement 5, pp. 22–38, 2012.
Barone A, Ricci M, Tonelli P, Santini S. Covani U. Tissue changes of extraction sockets in humans: a comparison of spontaneous healing vs. ridge preservation with secondary soft tissue healing. Clin. Oral Impl. Res. 24, 2013, 1231–1237 doi: 10.1111/j.1600-0501.2012.02535.x
Engler-Hamm, D., Cheung, W.S., Yen, A., Stark, P.C. & Griffin, T. (2011) Ridge preservation using a composite bone graft and a bioabsorbable mem- brane with and without primary wound closure: a comparative clinical trial. Journal of Periodontol- ogy 82: 377–387.
Trombelli, L., Farina, R., Marzola, A., Bozzi, L., Lil- jenberg, B. & Lindhe, J. (2008) Modeling and remodeling of human extraction sockets. Journal of Clinical Periodontology 35: 630–639.
Scarano, A., Piattelli, A., Assenza, B., Quaranta, A.,
Perrotti, V., Piattelli, M. & Iezzi, G. (2010) Por- cine bone used in sinus augmentation procedures: a 5-year retrospective clinical evaluation. The International Journal of Oral and Maxillofacial Surgery 68: 1869–1873.
bone grafting alone:
Froum S, Cho SC, Rosenberg E, Rohrer M, Tarnow D. Histological comparison of healing extraction sockets implanted with bioactive glass or demineralized freeze-dried bone allograft: A pilot study.
J Clin Periodontol 2002;73:94–102.
23. Zubillaga G, Von Hagen S, Simon BI, Deasy MJ. Changes in alveolar bone height and width following post-extraction ridge augmenta- tion using a xed bioabsorbable membrane and demineralized freeze-dried bone osteoinductive graft. J Periodontol 2003;74: 965–975.
xenograft:
Artzi Z, Nemcovsky CE. The application of deproteinized bovine bone mineral for ridge preservation prior to implantation. Clinical and histological observations in a case report. J Periodontol 1998; 69:1062–1067.
25. Carmagnola D, Adriaens P, Berglundh T. Healing of human extrac- tion sockets lled with Bio-Oss. Clin Oral Implants Res 2003;14: 137–143.
alloplast:
Sàndor GK, Kainulainen VT, Queiroz JO, Carmichael RP, Oikarinen KS. Preservation of ridge dimensions following grafting with coral granules of 48 post-traumatic and post-extraction dento-alveolar defects. Dent Traumatol 2003;19:221–227.
27. Guarnieri R, Pecora G, Fini M, et al. Medical grade calcium sulfate hemihydrate in healing of human extraction sockets: Clinical and histological observations at 3 months. J Clin Periodontol 2004;75:902–908.
only reabsorbable membranes:
Lekovic V, Camargo PM, Klokkevold PR, et al. Preservation of alveo- lar bone in extraction sockets using bioabsorbable membranes.
J Clin Periodontol 1998;69:1044–1049.
Only non reabsorbable membranes:
Diès F, Etienne D, Abboud NB, Ouhayoun JP. Bone regeneration in extraction sites after immediate placement of an e-PTFE membrane with or without a biomaterial. A report on 12 consecutive cases. Clin Oral Implants Res 1996;7:277–285.
Bartee BK. Extraction site reconstruction for alveolar ridge pres- ervation. Part 2: Membrane-assisted surgical technique. J Oral Implantol 2001;27:194–197.
membrane + graft
Iasella JM, Greenwell H, Miller RL, et al. Ridge preservation with freeze-dried bone allograft and a collagen membrane compared to extraction alone for implant site development: A clinical and histologic study in humans. J Clin Periodontol 2003;74:990–999.
Liberati A, Altman DG, Tetzla J; The Prisma Group. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Br Med J 2009;339:b2700.
Blanco, J., Alves, C.C., Nunez, V., Aracil, L., Munoz, F. & Ramos, I. (2010) Biological width following immediate implant placement in the dog: Flap vs. Flapless surgery. Clinical Oral Implants Research 21: 624–631.
Caneva, M., Botticelli, D., Salata, L.A., Souza, S.L.S., Bressan, E. & Lang, N.P. (2010) Flap vs. “flapless” surgical approach at immediate implants: a histomorphometric study in dogs. Clinical Oral Implants Research 21: 1314–1319.
Engler-Hamm, D., Cheung, W.S., Yen, A., Stark, P.C. & Griffin, T. (2011) Ridge preservation using a composite bone graft and a bioabsorbable mem- brane with and without primary wound closure: a comparative clinical trial. Journal of Periodontol- ogy 82: 377–387.
Darby, I., Chen, S.T. & Buser, D. (2009) Ridge pres- ervation techniques for implant therapy. International Journal of Oral and Maxillofacial Implants 24: 260–271.
Barone, A., Ricci, M., Calvo-Guirado, J.L. & Covani, U. (2011) Bone remodelling after regen- erative procedures around implants placed in fresh extraction sockets: an experimental study in Beagle dogs. Clinical Oral Implants Research 22: 1131–1137.
Barone A, Borgia V, Covani U, Ricci M, Piattelli A, Iezzi G. Flap versus flapless procedure for ridge preservation in alveolar extraction sockets: a histological evaluation in a randomized clinical trial. Clinical Oral Implants Research 00, 2014, 1–8 doi: 10.1111/clr.12358
Hammerle, C.H., Arau jo, M.G. & Simion, M. (2012) Osteology Consensus Group 2011. Evidence- based knowledge on the biology and treatment of extraction sockets. Clinical Oral Implants Research, 23(Suppl. 5): 80–82.
Sbordone, L., Levin, L., Guidetti, F., Sbordone, C., Glikman, A. & Schwartz-Arad, D. (2011) Apical and marginal bone alterations around implants in maxillary sinus augmentation grafted with autogenous bone or bovine bone material and simultaneous or delayed dental implant posi- tioning. Clinical Oral Implants Research 22: 485–491.
Becker, W., Dahlin, C., Lekholm, U., Bergstrom, C., van Steenberghe, D., Higuchi, K. & Becker, B. E. (1999) Five-year evaluation of implants placed at extraction and with dehiscences and fenestra- tion defects augmented with eptfe membranes: results from a prospective multicenter study. Clinical Implant Dentistry & Related Research 1: 27–32.
Artzi, Z., Tal, H. & Dayan, D. (2000) Porous bovine bone mineral in healing of human extraction sockets. Part 1: Histomorphometric evaluations at 9 months. Journal of Periodontology 71: 1015– 1023.
Carmagnola, D., Adriaens, P. & Berglundh, T. (2003) Healing of human extraction sockets filled with bio-oss. Clinical Oral Implants Research 14: 137–143.
Vignoletti F, Matesanz P, Rodrigo D, Figuero E, Martin C, Sanz M. Surgical protocols for ridge preservation after tooth extraction. A systematic review.
Clin. Oral Impl. Res. 23(Suppl. 5), 2012, 22–38 doi: 10.1111/j.1600-0501.2011.02331.x
Fickl S, Schneider D, Zuhr O, Hinze M, Ender A, Jung RE, Hu ̈rzeler MB. Dimensional changes of the ridge contour after socket preservation and buccal overbuilding: an animal study. J Clin Periodontol 2009; 36: 442–448. doi: 10.1111/j.1600-051X.2009.01381.x.
Ridge preservation techniques for implant therapy. Darby I, Chen ST, Buser D. Int J Oral Maxillofac Implants. 2009; 24 Suppl():260-71.)

