When the Italian soccer team plays a game, everyone, even who does not know anything about sport, becomes a soccer supporter: the day after the match, you can not go to the bar without knowing the result so you can discuss it with friends and colleagues! In the last few years, we have seen a similar phenomenon in the field of all-ceramic prostheses: everyone’s talking about them, everyone uses them, everyone claims to be experienced with them, even if they don’t really know a thing about them! Of course, nowadays, a dental surgeon can not do anything but use the most innovative materials and most up-and-coming techniques…!
However, confusion on this matter is widespread, testified by the fact that during courses and conferences there is not even any clarity concerning the terminology. What name should be given to integral ceramics? Alumina or zirconia? Alumina and zirconia? Some say they are oxides, but doesn’t an oxidized material suggest ideas of something that is used up, damaged or, in any case, not excessively resistant? Well, let’s clarify the ideas.
The use of gold and metallic alloys in prosthodontics is almost disappearing, thanks to the introduction of “white” materials whose aesthetic results are without doubt more attractive. The possibility to eliminate the visibility of unattractive metallic edges is a very pleasing solution, both for dentists and patients. And today integral ceramics allow both to plan new rehabilitations and re-operate on previous work that maybe does not satisfy the ever more pressing aesthetic demands.
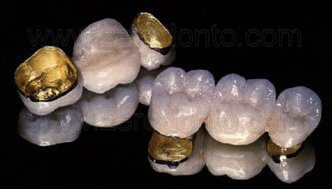
Figure 1 – Gold-ceramic fixed partial prosthesis.

Figure 2 – Zirconia-ceramic fixed partial prosthesis.

Figure 3 – Gold inlays and onlays in mandibular arch.

Figure 4 – Full-arch zirconia ceramic bridge.
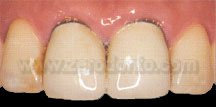
Figure 5 – Case study 1: Gold ceramic crowns on dental support in 11 and 21 areas. Exposure of the metallic borders on the vestibular surface with consequent cosmetic failure of the rehabilitation.

Figure 6 – Case study 1: Aesthetic resolution of the case through substitution of the incongruous restorations with alumina-ceramic crowns in 11 and 21 areas.

Figure 7 – Case study 2: Gold ceramic crown on implant support in area 21. Visibility of the metallic border on the vestibular surface with consequent cosmetic failure of the rehabilitation.
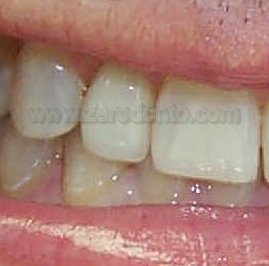
Figure 8 – Case study 2: Aesthetic resolution of the case through substitution of the incongruous restoration with alumina-ceramic crown in area 21.
However, all-ceramics are not properly ceramics, they are ceramic oxides of common metals. In our daily experience, we often come into contact with the silver colour of aluminium in cans, doors and tools. Zirconium instead has a white-greyish colour and is mainly used in the production of refractory materials, such as the infrastructure of nuclear reactors and even as precious synthetic stone. In the latter context, our female colleagues are surely experts in being able to accept or refuse the advances of a suitor according to whether a diamond or zircone is on offer!
Treated suitably, these aluminium and zirconium metals can be oxidized, thereby modifying radically their characteristics, starting from their names. Aluminium oxide (Al2O3) and zirconium oxide (ZrO2) are respectively called ‘alumina’ and ‘zirconia’. So, the first concept is clear now: in dental surgery it is never correct to use the term ‘zirconium’. Also, the aesthetic characteristics change deeply: from grey metallic to a more or less opaque white colour. Both these materials are very much appreciated in the biomedical field as they are biocompatible and have a high degree of hardness and resistance to the use.
This excellent mechanical resistance is not peculiar to alumina and zirconia but is rather the outcome of very sophisticated procedures of working. From a chemical-physical point of view, these materials are constituted of polycrystal structures but in their raw form, before industrial working, they have voids and microscopic defects that make them friable lending them a chalky consistency. Successively, thanks to a sintering process (high temperature firing under high pressure) alumina and zirconia take on the mechanical characteristics described before thanks to the elimination of the voids and defects of the polycrystal structure.
Zirconia has a phase modification that makes it particularly suited to prosthetization of the lateral posterior sectors. This material is stable in the monoclinic crystal form in which, in layman’s terms, its crystals take on the form of rounded pebbles. The industry, however, is able to block the structure of the material when the crystals have tetragonal form, similar to a pyramidal structure. The stopping of this phase transformation – irreversible and local in that it may affect only certain parts – is obtained through the application during the cooling phase after sintering of stabilizer oxides, essentially magnesium or yttrium. The acronym Y-TZP is used to define many types of zirconia used in dentistry (Yttrium Tetragonal Zirconia Polycrystals).
However, why does a further process of industrial working have to be made, given that as we said after sintering the zirconia obtains optimal values of mechanical resistance? Simple: because, being a ceramic oxide, the material withstands very well to compressive stresses but very much less satisfactorily to tensive strains, as do indeed all traditional ceramics. This means that during intraoral functions, zirconia structures can be subjected to forces and loads that may deteriorate their mechanical characteristics.
Some may think that a prosthesis undergoes tensional stress only if it is subjected to the action of a hammer or to the chewing of non suitable foods after a prosthetic rehabilitation, such as tough chewing gum, hard candy or liquorice sticks. However, any dentist knows perfectly well that patients nearly always attribute the fractures of restorations to the poor skill of the dentist or to the action of apparently “innocuous” foods like bread crumbs, minestrone or liver pâté!
Therefore, an explanation to this mysterious phenomenon must therefore be found …
No sooner said than done! Intraorally, the axial loads are a sort of chimera: there is no need to have a degree in physics to understand that the same inclination of the cuspidal planes and the dental roots makes the application of coaxial forces to the longitudinal axis of dental elements almost impossible. So, any force during chewing or other functional and parafunctional activities is constituted at least by an eccentric component that becomes an extra axial strain on the dental elements, be they natural or prosthetizated. In almost all cases, extra axial load means tensive stress. Bingo!
Using materials able to tolerate similar strains satisfactorily thus becomes increasingly important in order to achieve the long-term success of a prosthesis. The use of the oxides stabilizers in order to halt the transition of zirconia phase above mentioned has precisely this purpose: making the material able, within limits, to self-resist.
In case of accumulation of stress that exceeds the structural resistance of the material, zirconia is able to use this “damaging” energy to trigger the passage from the tetragonal form to the monoclinic one of the crystals (called ‘transformation toughening’) thereby determining a volumetric increase of the material equal to 4% that produces a compressive stress inside the material. This phenomenon allows zirconia not only to block the propagation of micro fractures inside the structure but to reduce their dimensions too.
However, it would be wise to recall that this “self-resilience” of zirconia can only happen once in a specific area of the material. In other words, the onset of a micro crack in a determined part of a prosthetic structure does not compromise the structural resistance of the whole framework but involves only and exclusively that area in which the accumulation of stress has occurred. Nevertheless, being an irreversible transition phase, once this phase has occurred in a specific area of the structure it cannot be repeated again in the same place and the material will inevitably result weakened.
Therefore, zirconia can ‘forgive’ or, to a degree, tolerate possible prosthetic planning errors or slight oversights in terms of control and maintenance over time of rehabilitations: file that under “Learning from mistakes”! But care must be taken not to abuse the patience of zirconia..!
Find out more here:
1. Zarone F., Sorrentino R., Vaccaro F., Traini T., Russo S., Ferrari M. Acid etching surface treatment of feldspathic, alumina and zirconia ceramics: a micro-morphological SEM analysis. International Dentistry South Africa, 2006; 8(3): 50-56.
2. Zarone F., Sorrentino R., Vaccaro F., Russo S., De Simone G. Retrospective clinical evaluation of 86 all-ceramic anterior single crowns on natural and implant-supported abutments. Clinical Implant Dentistry and Related Research, 2005; 7(Suppl 1):S95-103.
3. Salameh Z., Sorrentino R., Ounsi H., Goracci C., Tashkandi E., Tay F.R., Ferrari M. Effect of different all-ceramic crown system on fracture resistance and failure pattern of endodontically treated maxillary premolars restored with and without glass fiber posts. Journal of Endodontics, 2007; 33(7): 848-851.
For information:
zerodonto@gmail.com
tel. 0039 081 2451805

