Bisphosphonate therapy and the manifestation of a rare and severe complication, namely the osteonecrosis of the maxillary bone, are currently very important topics to which increasing attention is being given by specialists. The dental surgeon’s awareness and concern toward this pathology, together with a degree of alarmism, is justified by the increasing number of cases that since 2003 till today have enabled observing and studying the clinical and anatomo-pathologic case history, the localization of the pathology, the associated risks and the possible development and consequences of the osteonecrosis lesions. Epidemiological data are more unclear as they do not actually consent defining the exact prevalence and incidence of the phenomenon, the pathogenic hypothesis and the causal relationship related to the use of the bisphosphonates.
Other uncertainties predominate the scenario of the therapeutic possibilities that seem inadequate to tackle the needs of patients affected by osteonecrosis of the maxillary bone. Till now, there is no therapy leading to resolution of the lesions, neither has the suspension of the therapy, in some cases, led to improve the effects.
In the light of these considerations, the goal that dental surgery must achieve is to arm itself with whatever means possible in order to at least reduce the negative side-effects of this symptoms, often painful, and the risks of bacterial or other microorganism contaminations which worsen the case history and compromise the general health of the patient. The aim of this article is to illustrate, as a schematic overview, the current knowledge on bisphosphonates.
Definition: Bisphosphonates are non-hormonal substances, active in mineral bone homeostasis. Structural analogues of inorganic pyrophosphates but resistant to enzymatic hydrolysis, bisphosphonates are molecules able, through a rigid bond with hydroxyapatite crystals, to modulate skeletal turnover and, in particular, selectively inhibit osteoclast mediated bone reabsorption.
|
Commercial products
|
Preparations
|
Agent
|
|
Adronate (Neopharmed)
|
Tablets
|
Alendronic acid
|
|
Alendronato Pliva (Pliva Pharma)
|
Tablets
|
|
|
Alendronate Ratiopharm (Ratiopharm GmbH-D)
|
Tablets
|
|
|
Alendronate Teva (Teva Pharma Italy)
|
Tablets
|
|
|
Alendros (Abiogen Pharma)
|
Tablets
|
|
|
Dronal (Sigma-Tau)
|
Tablets
|
|
|
Dronal 70 (Sigma-Tau)
|
Tablets
|
|
|
Fosamax (Merck Sharp Dohme)
|
Tablets
|
|
|
Genalen (Gentili)
|
Tablets
|
|
|
Etidron (Abiogen Pharma)
|
Tablets
|
Etidronic
Acid
|
|
Bondronat (Roche Registration – GB)
|
Tablets
|
Ibandronic
Acid
|
|
Bonviva (Roche Registration – GB)
|
Tablets
|
|
|
Aredia (Novartis Farma)
|
Intravenous drug
|
Pamidronic Acid
|
|
Amidrox (Crinos)
|
Intravenous drug
|
|
|
Pamidronate Disodium IBP
Pharma (IBP Pharma)
|
Intravenous drug
|
|
|
Texpami (Pharmatex Italia)
|
Intravenous drug
|
|
|
Pamidronate Disodium Mayne (Mayne Pharma Italy)
|
Intravenous drug (for hospital use only)
|
|
|
Actonel (Procter & Gamble)
|
Tablets
|
Risedronic
Acid
|
|
Optinate (Lepetit)
|
Tablets
|
|
|
Clodronic Acid EG (Eg)
|
Intravenous drug
|
Clodronic
Acid
|
|
Clodronic Acid Sandoz (Sandoz)
|
Intravenous drug
|
|
|
Clodronic Acid
Union Health (Union Health)
|
Intravenous drug
|
|
|
Clasteon (Abiogen Pharma)
|
Tablets/phials
|
|
|
Climaclod (Mastelli)
|
Intravenous drug
|
|
|
Clodeosten (B&G)
|
Intravenous drug
|
|
|
Clodron (Fidia pharmaceuticals)
|
Tablets/phials
|
|
|
Clodronate ABC (ABC pharmaceuticals)
|
Intravenous drug
|
|
|
Clodronate Teva (Teva Pharma Italia)
|
Intravenous drug
|
|
|
Clody (Promedica)
|
Tablets/phials
|
|
|
Difosfonal (SPA)
|
Intravenous drug
|
|
|
Disodium Clodronate Alter(Alter)
|
Intravenous drug
|
|
|
Moticlod (Lisapharma)
|
Intravenous drug
|
|
|
Niklod (Savio I.B.N.)
|
Intravenous drug
|
|
|
Osteonorm (Piam)
|
Intravenous drug
|
|
|
Osteostab (Rottapharm)
|
Intravenous drug
|
|
|
Soclonat (TB Technology)
|
Intravenous drug
|
|
|
Aclasta (Novartis Europh-GB)
|
Intravenous drug
|
Zolendric
Acid
|
|
Zometa (Novartis Europh-GB)
|
Intravenous drug
|
|
Bone binding affinity and biological potency
The antireabsorptive potency of bisphosphonates depends on the bone binding affinity and their ability to inhibit osteoclast action. The binding affinity of various bisphosphonates to the bone depends on the molecular group in the R1 side chain and is increased by the presence of a group – OH or – NH2 in that position. The different affinities of the bisphosphonates influence the drug quantity accumulated, the persistence of the bone matrix and the duration of action.
In increasing order of affinity:
Clodronate, etidronate, pamidronate, risedronate, ibandronate, alendronate, zolendronate [3]
The potency of inhibition of the osteoclast action depends on the molecular group in R2. Bisphosphonates with a -NH2 group in that position (amino-bisphosphonate) are more potent than those lacking this group (non amino-bisphosphonate) as they are able to arrest the osteoclast action through the inhibition of mevalonic acid pathway (see next paragraph). The potency is also related to the three-dimensional conformation of the R2 chain and therefore, to the length of the chain and to the introduction of methyl, pentilic, imidazole and benzene groups.
In increasing order of potency:
Etidronate, clodronate, pamidronate, alendronate, ibandronate, risedronate, zolendronate [4]
Biological potency and binding affinity are characteristics closely related to each other in establishing the real anti-reabsorptive potency in vivo. Bisphosphonates with high binding affinity to the mineralized surface are held within the bone to a greater degree and have greater pharmacological effects. Furthermore, the bone matrix functions like a sponge, able to absorb, release locally and reabsorb the bisphosphonates to a greater degree the higher the affinity is. Therefore, this would explain the persistence of the pharmacological effects observed after the administration of bisphosphonates [2]. From the analysis of two schemes, it appears that zolendronate is the drug with the highest affinity and potency, while alendronate has greater potency in inhibition of the bone turnover in comparison to risedronate.
Mechanism of action
Thanks to their high affinity with the hydroxyl-apatite, bisphosphonates firmly bound to the bone crystals, are released in the extracellular space during the phase of bone reabsorption and are then absorbed by endocytosis into the osteoclasts. Inside these cells, the non-amino bisphosphonates (not having aminic terminal groups in the side chain bound to the C atom in position 1- etidronate and clodronate) are metabolized in non-hydrolysable analogues of the ATP with consequent cytotoxic effect and death of the cells. On the other hand, the amino-bisphosphonates (alendronate, risedronate, ibandronate, pamidronate and zolendronate) more potent since having – NH2 groups in the side chain R2, selectively inhibit the farnesyl pyrophosphate synthase enzyme implicated in the biosynthesis of the sterols (cycle of the mevalonic acid), and prevent the prenilation of some GTP-ase dependent proteins involved in the cell cycle maintenance with consequent cytoskeleton alteration and cell death by apoptosis [1].
Chemical structure
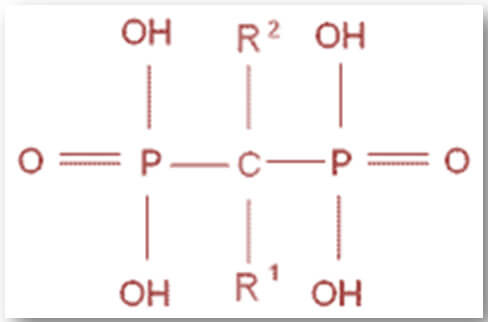
Indications
Prevention of the brittle fractures during chronic diseases:
The use of these drugs, aimed at a reduction in bone turnover, does not however have a curative purpose and is prolonged over time. The reduction of the bone turnover allows the formation of more mineralized and dense bone and reflects in an increase of the mineral bone density. However, an excessive slowdown of the bone remodelling is in turn dangerous for two reasons: 1. the bone, not being able anymore to repair damage by use, may be subject to brittle fracture owing to accumulation of microcracks; 2. an over mineralized bone also results more fragile because it is not elastic [5]).
- Postmenopausal Osteoporosis; Male Osteoporosis
- Paget’s disease
- Osteogenesis imperfecta
- Hypercalcemia and malignant neoplasias
- Bone Metastases
Osteonecrosis of the maxilla: complications
Osteonecrosis may occur in patients receiving intravenous (zolendronate and/or pamidronate) or oral bisphosphonates (alendronate, risendronate and ibandronate for chronic use) [6].
The first case report dates back to 2003 [7].
Definition: Disabling complication regarding the maxillary and mandibular bone due to accumulation of bisphosphonates in these tissues.
Localizzation:
- mandible 65%
- maxilla 26%
- mandible + maxilla 9% [8].
Clinical evaluation
Symptoms may be silent for weeks or months until bone exposure in oral cavity associated to bacterial infection or even trauma of the surrounding soft tissues [8,9].
Signs and symptoms:
- Localized pain
- Soft-tissue edema
- Bleeding
- Paresthesia
- Soft tissue infections
- Cutaneous fistulas.
Prodromic manifestations:
- Alterations of the periodontal mucosa
- Altered healing process of the oral mucosa
- Oral mucosa infections
Radiographic signs: The radiographic evidence- verifiable only in advanced stages of the pathology – shows an area of osseous rarefaction. At the same time it is sometimes possible to observe radiopaque areas related to the bone sequestrations
Anatomic pathologic signs:
Macroscopic report: bone tissue of white-yellowish colour, non bleeding, with possible fistulisation towards the cutis and surrounded by inflamed mucosal tissues.
Histological signs: confluent areas of necrotic tissue, alternating with healthy tissue, without signs of bacterial invasion and with conservation of the vascular channels. Presence of inflammatory tissue, bacteria, and numerous osteoclast cells (often not active) in the areas surrounding the lesion.
Epidemiology: despite the increasing number of cases reported in dental literature, it is still not possible to estimate the precise incidence. However, a time and dose-dependent trend has been evaluated [10].
Median time of appearance: between 22 and 39 months from the beginning of the bisphosphonate treatment, with shorter times for zolendronate than pamidronate [8].
Why the maxillary bones? The accumulation of bisphosphonates at the maxillary bones seems to be partly due to the rich vascularizzation and high bone turnover that characterize these bone segments subjected to continuous mechanical stress and also to the binding affinity of a specific bisphosphonate to the hydroxyapatite [11].
Possible consequences: osteomyelitis due to bacterial contamination.
Risk factors:
- Active principle: All cases of osteonecrosis of the maxillary bone reported in the literature are associated with amino-biphosphonate use
The strong association (94%) between the appearance of osteonecrosis of the maxillary bone and the administration of zolendronate and/or pamidronate is well known, depends on the highly potent action of these drugs in suppressing bone turnover and on the particular antiangiogenetic effects of the zolendronate [12]. However some cases associated with the use of alendronate via oral administration (4.2%), alendronate in association with zolendronate (0.6%), risedronate via oral administration (0.3%), risendronate in association with zolendronate (0.3%) and ibandronate via oral administration(0.3%) have also been documented.
- Dosage and administration [13]
(The effective dose for the bone tissue depends in turn on the way of administration of the drug. Intravenous and intramuscular administration ensure a 100% bioavailability, while oral administration is characterized by a low bioavailability (0.5-2% absorption of the drug taken).Administration of high-dosage intravenous zolendronate and pamidronate for treating bone metastasis is associated with the high prevalence of osteonecrotic lesions.)
- Duration of the treatment
(The risk of osteonecrosis of the maxillary bone increases in patients treated for more than 12 months with diphosphonates [14])
- in metastatic bone disease, the incidence of the osteonecrosis of the maxillary bone is 10% versus 0.7-1 cases per 100.000 patients/year affected by osteoporosis [15]
- Iatrogenic or spontaneous traumatism in oral tissues [16]
(Literature reports the strong association (60-85% of the cases) between the appearance of osteonecrosis of the maxillary bone and previous invasive orthodontic interventions (such as extraction of a dental element, implantology), or the use of incongruous dental prosthesis, with mean time of appearance between 3 and 12 months. However, it is possible that a subclinical form of the illness hides behind a dental pathology [17])
- Dental pathologies (present in 29% of the cases) and acute and chronic periodontal diseases (present in 84% of cases) that may allow the passage of bacteria across the alveolar cavity
- Concomitant pathological conditions
(Patients with immune-deficiencies, diabetes, neoplasia or in therapy with corticosteroid, immunosuppressors and antineoplastic drugs, have a high risk of developing osteonecrosis of the maxillary bone, but this has still not been quantified [18])
Management of patients receiving bisphosphonate treatment
|
Patients not yet treated with amino-bisphosphonates or with three months or less of therapy
|
Amino-bisphosphonate-treated patients with more than three months of therapy in which there is no evidence of osteneocrosis of the maxillary bone
|
|
Oral cavity health evaluation
|
Maintenance of good oral hygiene
|
|
Panoramic radiographic examination
|
Frequent checks of the prosthetic devices in order to avoid traumatic lesions of the soft tissues
|
|
Endoral radiography status
|
Avoid make surgical interventions except when indispensable, by local and systemic antibiotics
|
|
Elimination of infectious centres
|
Perform non invasive therapy instead of surgical treatments, by local and systemic antibiotics
|
|
Periodontal treatment
|
|
|
Conservative and endodontic treatment of compromised teeth
|
|
|
Extraction of non-recoverable teeth
|
|
|
|
Treatment of patient affected by osteonecrosis of the maxillary
Given that there is no appropriate therapy that can assure resolution of the pathology until now (even suspending bisphosphonate treatment does not benefit patients affected by osteonecrosis of the maxillary bone because of the long term bioavailability and the systemic uptake of these drugs [20]), the goal that doctors and dental surgeons must pursue should be the pain reduction and control of secondary infections in the necrotic areas. For this reason, the daily application of antimicrobic (Chlorhexidine 0.12%) or anti-inflammatory drugs is recommended and the prescription of systemic antibiotic therapy (b-lattamines or macrolids in case of allergy to penicillin and metronidazol) and anti-micotic therapy in case of local infections [19]. If necessary, the conservative removal of necrotic bone is recommended, with minimum trauma to the adjacent tissues. This removal aims to reduce traumas to the tissues surrounding the lesion caused by the sharp edges of the necrotic bone.
References
[1] Papapoulos SE (2006) Bisphosphonate actions: physical chemistry revisited. Bone 38:613-616.
[2] Fiore C.E., Pennisi P. (2006) Bifosfonati a confronto: farmacologia. Bifosfonati 2: 13-22.
[3] Nancollas GH, Tang R, Phipps RJ et al (2006) Novel insights into actions of bisphosphonates on bone: differencesin interactions with hydroxyapatite. Bone 38:617-627.
[4] Roelofs A, Thompson K, Gordon S, Rogers MJ (2006) Molecular mechanisms of action of bisphosphonates: current status. Clin Cancer Res 12:6222-62.
[5] Filipponi P., Cristallini S., Policani G. (2006) Bifosfonati: effetti a lungo termine. Bifosfonati 8: 63-76.
[6] Ruggiero SL et al. (2004) Osteonecrosis of the jaws associated with of biphosphonates: a review of 63 cases. J Oral Maxillofacial Surg 65: 527-534.
[7] Marx RE (2003) Pamindronate (Aredia)and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg 61: 115-117.
[8] Woo S, Hellstein JW, Kalmar JR (2006) Narrative [corrected] review: bisphosphonates and osteonecrosis of the jaws. Ann Intern Med 144:753-761.
[9] Van den Wyngaert T, Huizing MT, Vermorken JB (2006) Bisphosphonates and osteonecrosis of the jaw: cause and effect or a post hoc fallacy? Ann Oncol 17:1197-1204.
[10] Ambrogini E., Marcocci C. (2006) Profilo di sicurezza dei bifosfonati. Bifosfonati 6: 47-56.
[11] Cremers SC, Papapoulos SE, Gelderblom H et al (2005) Skeletal retention of bisphosphonate (pamidronate) and its relation to the rate of bone resorption in patients with breast cancer and bone metastases. J Bone Miner Res 20:1543-1547.
[12] Woo S, Hellstein JW, Kalmar JR (2006) Narrative [corrected] review: bisphosphonates and osteonecrosis of the jaws. Ann Intern Med 144:753-761
[13] Berenson JR, Rosen LS, Howell A, Porter L, Coleman RE, Morley W, et al. Zoledronic acid reduces skeletal-related events in patients with osteolytic metastases, Cancer 2001;91:1191-200.
[14] Durie BG, Katz M, Crowley J, Osteonecrosis of the jaw and bisphosphonates. N Engl J Med 2005;353(1):99-102.
[15] Bamias A, Kastritis E, Bamia C, Moulopoulos LA, Melakopoulos I, Bozas G, Koutsoukou V, Gika D, Anagnostopoulos A, Papadimitriou C, Terpos E, Dimopoulos MA.
Bilezikian JP (2006) Osteonecrosis of the jaw – Do bisphosphonates pose a risk? N Engl J Med 355:2278-2281.
[16] Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: incidence and risk factors. J Clin Oncol 2005, 23(34):8580-7.
[17] Dunstan C, Felsenberg D, Seibel MJ (2007) Therapy insight: the risks and benefits of bisphosphonates for the treatment of tumor-induced bone disease. Nat Clin Pract Oncol 4:42-55.
[18] Woo SB, et al. Systematic review: Bisphosphonates and osteonecrosis of the jaws. Ann Intern Med 2006, 144:753-761.
[19][20] Marx RE, et al. Bisphosphonateinduced exposed bone (osteonecrosis/osteopetrosis) of the jaws: risk factors, recognition, prevention, and treatment. J Oral Maxillofac Surg 2005, 63:1567-1575.
Other references:
Katzung B. G., Piccin-Nuova Libraria-2006 Farmacologia generale e clinica.
Aggiornamento in tema di Bifosfonati, Organo ufficiale del GIBIS Gruppo Italiano Per Lo Studio Dei Bifosfonati, Yearbook 2006, casa editrice Springer.
Bertoldo F.,Dalle Carbonare L., Pancheri S., Lo Cascio V., Nocini P.F. L’osteonecrosi della mandibola associata alla terapia con bofosfonati, Selezione Abstract, Vol. VIII n^ 2, maggio 2007.
For further info:
Dr. Natascia Raciti
natasciaraciti@gmail.com

